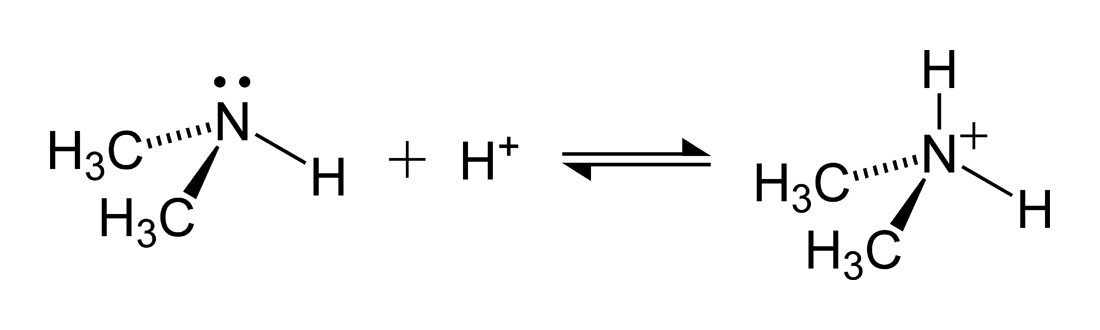Concept Question
Which of the following is not a strong base?
(touch choices to toggle feedback on/off)
Brønsted-Lowry Definition
The Brønsted-Lowry definition of acids and bases is that:
An acid is a PROTON donor
A base is a PROTON acceptor.
A PROTON is an "H+" which is a hydrogen without an electron. The H+ swaps places between two different molecules (the donor acid and the acceptor base).
This idea that the proton is swapping place is a powerful way to view chemistry. Acid/Base compounds can be found in one of two forms. Either with the proton on the molecules, "the protonated form". Or with the proton off the molecule, "the de-protonated" form". The protonated form of the molecule is the acid form. The deprotonated form is the base form. Therefore each compound can be both an acid and a base depending on whether it is protonated or not. Such protonated/de-protonated pairs are known as conjugated pairs. It is important to be able to recognize such pairs as they can interchange simply by the adding or subtracting a proton. Whether we typically refer to a compound as an acid or base depends a great deal on the relative acid/base strength of the compounds (as will be seen later). But it is critical to realize that all acid base compounds are conjugate pairs from the Bronsted-Lowry perspective.
For example, a simple acid is acetic acid CH3COOH
Acetic acid can be either "protonated" as shown on the left or it can lose a proton and be "de-protonated" as is shown on the right. The de-protonated form is called the acetate ion. We will deal with naming compounds more later. For now, you should simply be able to identify that these two compounds are conjugate pairs as they differ by only one proton. The acetate ion is the conjugate base of acetic acid. Acetic acid is the conjugate acid of the acetate ion.
If we put acetic acid in water, some of the acid will donate a proton to water to form the hydronium ion, H3O+. In this case, acetic acid is the acid as it is donating the proton. Water is the base as it is accepting the proton. The acid converts to its conjugate base, the acetate ion. The base converts to its conjugate acid, the hydronium ion.
CH3COOH(aq) + H2O(l) ⇌ CH3COO- (aq) + H3O+(aq)
Every acid base reaction will have a reactant that is an acid and a reactant that is a base. This is what defines acid/base chemistry. In the Bronsted-Lowry defnition, these two will be exchanging a proton to generate their conjugate partners.
A video illustrating the definition of Bronstead-Lowry Acids and Bases. Examples are given of each type.
Concept Question
In the following reaction, which compound is the proton donor (for the forward reaction)?
\( {\rm NH_3(aq) + H_2O(l) \rightleftharpoons NH_4^+(aq) + OH^-(aq)}\)
(touch choices to toggle feedback on/off)
Weak acids and weak bases
A weak acid is a proton donor that when put in water will only partially dissociate.
A weak base is a proton acceptor that when put in water will only partially dissociate.
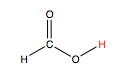
Let's look at the example of a weak acid, formic acid, HCOOH. This is a carboxylic acid that has the following structure. The acidic hydrogen is the one that is labelled in red. The electronegativity of the oxygens pull the electron density away from the hydrogen and leads to its partial ionization in solution.
Thus the equilibrium constant for this reaction
\[\rm{HCOOH(aq) + H_2O(l) \rightleftharpoons HCOO^-(aq) + H_3O^+(aq)}\]
is assumed to be small. We always write the equilibrium for a weak acid the same way. One mole of the acid plus one mole of water forms the hydronium ion (H3O+) and the deprotonated acid (the ion that is the acid minus the proton). We give this equilibrium constant a special name and call it Ka where the small a stands for acid (much like the sp in Ksp stood for solubility product). It is not different than any other equilibrium constant. It simply helps us to remember it is for this type of reaction written in this specific form. Thus the equilibrium constant will be
\[K_{\rm a} = {{\rm [H_3O^+][HCOO^-]} \over {\rm [HCOOH]}}\]
The "strength" of the weak acid will depend on the size of this equilibrium constant. The larger Ka is, the more it favors the dissociated side of the reaction. The smaller Ka, the less it favors the dissociated side of the reaction. For all weak acids, Ka is generally smaller than 1. That means for all weak acids the reactants are always favored. So if you put a weak acid in water the protonated form will have a higher concentration than the deprotonated form.
You'll also note that in this reaction the acid HCOOH is "changing" forms between having the proton HCOOH and not having the proton HCOO-. We can link these two species as what we could call "conjugate pairs". The protonated form HCOOH is the acid and the deprotonated form HCOO- is the conjugate base. That is formic acid (HCOOH) and the formate ion (HCOO-) are a conjugate pair. In the same reaction, the water is the base (deprotonated) while H3O+ (the protonated form) is the conjugate acid.

The base here is the deprotonated form of the acid
We can also write similar expressions for a base. Take ammonia. When it is placed in water it accepts a proton from water
\[\rm{NH_3(aq) + H_2O(l) \rightleftharpoons NH_4^+(aq) + OH^-(aq)}\]
Again NH3 is the base (deprotonated) and its conjugate acid is NH4+. The equilibrium constant for this reaction is written as Kb
\[K_{\rm b} = {\rm {[NH_4^+][OH^-]} \over {[NH_3]}}\]
the strength of the base will depend on the magnitude of Kb. The larger Kb the stronger the base.

The acid here is the protonated form of the base
>A video on Weak Acids and Weak Bases.
Concept Question
Which of the following is a weak acid?
(touch choices to toggle feedback on/off)
Identifying Weak Acids and Bases
One of the tripping points for many students in acid/base problem is simply identifying compounds as acids or bases. Step 1 in the process is to memorize the strong acids and strong bases. Next you need to be able to recognize the weak acids and bases. One of the best ways to do this is to learn something about the names and the structures of the major classes of acid/base compounds. Note we are pairing up compounds based on structures and looking at the conjugate acid base pairs.
Carboxylic Acids
Carboxylic acids and their conjugate bases are among the most important compounds to be able to recognize as they are extremely important in organic and biochemistry.
Carboxylic acids have a carboxylic acid group, a carbon double bonded to an oxygen also bonded to an oxygen with a hydrogen. It is this hydrogen that is the acidic hydrogen. When the proton is removed, the remaining ion is called a carboxylate.
This is shown below for acetic acid.
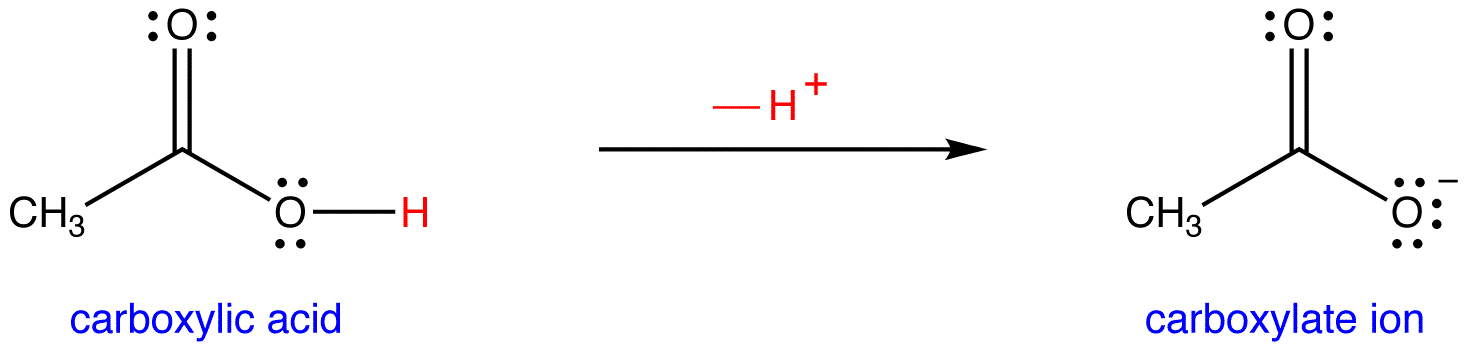 You can identify the compounds either by their chemical structure or their name. When written out, the formula for a carboxylic acid is given as COOH and a carboxylate as COO-. The names also follow these same patterns. The acid has the name 'something-ic acid.' The conjugate base has the name 'something-ate.'
You can identify the compounds either by their chemical structure or their name. When written out, the formula for a carboxylic acid is given as COOH and a carboxylate as COO-. The names also follow these same patterns. The acid has the name 'something-ic acid.' The conjugate base has the name 'something-ate.'
Here a few carboxylic acid names along with the names of their conjugate base ions.
formic acid, HCOOH the formate ion HCOO-
acetic acid, CH3COOH the acetate ion CH3COO-
benzoic acid, C6H5COOH the benzoate ion C6H5COO-
Amines
The next class of compounds that is important to recognize are amines and their conjugate acids. These compounds are essentially derivatives of ammonia (NH3) and its conjugate acid the ammonium ion (NH4+).
These compounds generally have the name 'something- amine' or 'something ammonium.'
They can be recognized by the chemical formula which will look like ammonia with either one hydrogen substituted for a carbon chain (primary), two hydrogens substituted (secondary), or three hydrogens substituted (tertiary). The corresponding conjugated acids will be the same, but with an added hydrogen and a positive charge
ammonia, NH3 the ammonium ion, NH4+
methyl amine , CH3NH2 the methyl ammonium ion, CH3NH3+
methyl ethyl amine, CH3NHC2H5 the methyl ethyl ammonium ion, CH3NH2C2H5+
Below is a picture of the weak base dimethyl amine accepting a proton to form the dimethylammonium ion.
Hydracids
The next class of compounds are hydracids. These are acids in which the proton is combined with an anion. In this case the name of the compound is 'something acid' and the corresponding conjugate base is simply the name of the anion that forms from deprotonation. For example there are several strong acids that are hydracids. The hydrochloric acid, HCl, has a corresponding conjugate base the chloride ion, Cl-. Since HCl is considered to be infinitely strong, Cl- is infinitely weak as a base. However, there are other compounds like hydrofluoric acid, HF and hydrocyanic acid HCN in which the conjugate acids anions are weak bases. In the case of HF, the conjugate base is the fluoride ion, F-. For cyanic acid, the weak base is then cyanide ion, CN-.
Oxoacids
Finally there are the oxoacids. These are protonated non-metal oxides. For example nitric acid, HNO3. The corresponding conjugate base is nitrate, NO3-. (like chloride since the acid is infinitely strong, the conjugate base is infinitely weak). There is a systematic way to name these acids and their corresponding conjugate base anions. First it is most important to be able to recognize them. The acids are non-metals bonded with oxygen and hydrogen. The conjugate bases are identical, but they are missing a proton (minus one hydrogen and they are anions).
Chlorous Acid, HClO2 the chlorite ion, ClO2-
Chloric Acid, HClO3 the chlorate ion, ClO3-
Carbonic Acid, H2CO3 the hydrogen carbonate ion, HCO3_
Concept Question
What is the conjugate acid of ammonia NH3?
(touch choices to toggle feedback on/off)
Spectator Ions
We also have the potential to have some ions in solution that do not participate in the chemistry. This is because these ions are the conjugate pairs for the strong acids or strong bases. Since those reactions are assumed to go 100% in the forward direction, we can likewise assume they go backwards 0%. This means that those ions will simply remain in solution.
What are these ions? Well you can simply look at the lists of strong acids and strong bases and pick the conjugates.
For example, the conjugate base for HCl would be Cl-. As such, in aqueous solution the chloride ion is often a spectator ion (when is this not the case, when it is part of a solubility equilibrium. As far as acid/base chemistry it is always a spectator). Other examples include Na+. It is the partner for OH- in the strong base NaOH. Since this dissociates 100%, we can assume it will not react at all with any OH-. Other examples are Nitrate, NO3-, which is the conjugate base of nitric acid. K+, Ba2+, ...
Look at the list of strong acids and bases for all of them.
Concept Question
Which of the following will not be a spectator ion in an acid/base reaction?
(touch choices to toggle feedback on/off)
Strong Acid Equilibria
We are often interested in solving strong acid (base) problems. While these compounds are involved in equilibria we typically simplify the problem by assuming the dissociation of strong acids is 100%. This is because their equilibrium constants are so large. For example, look at the reaction of hydrochloric acid and water.
\[\rm{HCl(aq) + H_2O(l) \rightleftharpoons H_3O^+(aq)+ Cl^-(aq)}\]
The equilibrium constant for this reaction is
\[K_{\rm a} = {\rm [H_3O^+][Cl^-] \over [HCl]}=1.3 \times 10^6\]
1.3 x 106 is a large number. This reaction greatly favors the products. As such, it is safe to assume that the reaction has formed essentially all the products that are possible from the amount of HCl put into the solution. The equilibrium constant would only be of interest if we wanted to know the exact concentration of the minute amount of HCl at equilibrium. Instead, the concentration of interest for acid solutions is the hydronium ion concentration,[H3O+]. This is simply equal to the initial concentration of the acid in the solution, CHA. Thus for strong acid solutions we have a simple relationship between the acid concentration and the hydronium ion concentration at equilibrium
\[ {\rm[H_3O^+]} \approx C_{\rm HA}\]
This is approximate since it relies on a couple of assumptions. First it assumes that the initial concentration of hydronium ion in pure water is zero. It is not. It is very very small, but it is not zero. Therefore this approximation gets worse the more dilute the solution. The other approximation is that the equilibrium doesn't matter as the reaction is 100% products. This approximation also gets worse as the solution is more dilute. For typical concentrations used in a laboratory situation, this approximation is excellent even to several decimal places.
The same is true for a strong base except that the relationship is between the concentration of the base and the hydroxide ion concentration
\[ {\rm[OH^-]} \approx C_{\rm B}\]
The only caveat here is that one needs to be careful with the strong bases that produce two moles of OH- for each mole of compound. In this case you need to remember to multiply the base concentration by a factor of two. A 1 M Ba(OH)2 solution will have [OH-] = 2 M.
Concept Question
What is the concentration of H3O+ in a 0.2M solution of HBr? (touch choices to toggle feedback on/off)
Weak Acid Equilibria
We are often interested in solving weak acid (base) equilbria problems. While there are a huge number of such potential applications, there is one type of problem in particular that comes up over and over again. These problems give some initial concentration of acid (base) and seek the concentrations of chemical species at equilibrium. In particular, for acid problems we are interested in the hydronium ion concentration, [H3O+]. For base problems, we are usually interested in the hydroxide ion concentration, [OH-].
Let's examine a typical weak acid problem. You'd like to know the hydronium ion concentration, [H3O+] for a 0.1 M solution of benzoic acid. Benzoic acid is a weak acid (Ka= 6.4 x 10-5) so you know that the concentration will be very low. This is an equilibrium problem so you'll want to look at both the chemical equation and the equilibrium constant. We write the same equation for every weak acid in water. The acid plus the water react to form the hydronium ion and the deprotonated acid (the benzoate ion).
\[\rm{C_6H_5COOH(aq) + H_2O(l) \rightleftharpoons C_6H_5COO^-(aq) + H_3O^+(aq)}\]
The equilibrium constant for this reaction is
\[{\rm K_a = {{[H_3O^+][C_6H_5COO^-]} \over {[C_6H_5COOH]}}}\]
We can find the [H3O+] at equilibrium if we know the initial concentration of benzoic acid and the Ka for benzoic acid. The initial concentration of the acid is 0.1 M. Ka= 6.4 x 10-5.
To solve this problem we can set up a RICE table. We know the initial concentration of benzoic acid is 0.1M. We will assume the concentrations of the other ions are zero (this is subtle approximation for the hydronium ion). We will choose as our variable "x" the concentration of the acid that dissociates.
| R | C6H5COOH | H2O | C6H5COO- | H3O+ |
| I | 0.1 | - | 0 | 0 |
| C | -x | - | +x | +x |
| E | 0.1-x | +x | +x |
Putting the equilibrium values into the mass action expression we can solve for the amount of acid that dissociated.
\[{\rm K_a = {{[H_3O^+][C_6H_5COO^-]} \over {[C_6H_5COOH]}}}= {{(x)(x)} \over (0.1-x)}\]
Since we know Ka this can be re-arranged to yield a quadratic equation that we can solve. However, we can very easily arrive at an approximate answer. This is because we know that "x" will be small. That is that it is a small number compared to the initial concentration. How do we know this? Benzoic acid is a weak acid. This means the equilibrium favors the reactants. Ka is small. As a result we know what the concentration of the acid will be at equilibrium. It will be essentially unchanged. As a result we can approximate the equilibrium concentration of (0.1-x) ≈ 0.1. Putting this and the value of Ka for benzoic acid into the equation we find.
\[{\rm K_a }= 6.4 x 10^{-5}= {{(x)(x)} \over (0.1-x)} \approx {{(x)(x)} \over (0.1)}= {x^2 \over 0.1}\]
This is simple to solve and we find
\[ x = \sqrt{(6.4 x 10^{-5})(0.1)} = 2.5x10^{-3}\]
Now it is important to remember how we defined "x". In this case it was the concentration change for the acid. It also happens that for this situation, the hydronium ion concentration [H3O+]=x.
This problem can be solved in the exact same manner for any weak acid in some concentration in pure water. If we call the weak acid HA with an initial concentration, CHA we can find the concentration of all the species at equilibrium if we know Ka for the acid.
\[\rm{HA(aq) + H_2O(l) \rightleftharpoons A^-(aq) + H_3O^+(aq)}\]
The equilibrium constant for this reaction is
\[{\rm K_a = {{[H_3O^+][A^-]} \over {[HA]}}}\]
Setting up the RICE table for an initial concentration of CHA for the weak acid HA. And defining the number of moles of the acid that dissociates as "x" yields.
| R | HA | H2O | A- | H3O+ |
| I | Ca | - | 0 | 0 |
| C | -x | - | +x | +x |
| E | Ca-x | +x | +x |
Putting this into the equilibrium expression we find
.\[{\rm K_a = {{[H_3O^+][A^-]} \over {[HA]}}}= {{(x)(x)} \over (C_{HA}-x)}\]
We can again make the assumption that the change in concentration of the acid is negligible. This gives
\[{\rm K_a }= {{(x)(x)} \over (C_{HA}-x)} \approx {{(x)(x)} \over (C_{HA})}= {x^2 \over C_{HA}}\]
Solving this we get
\[ x = \sqrt{{\rm (K_a)(C_{HA})}}\]
Remembering that we defined "x" as the change in concentration of the weak acid we know that this is also the concentration of the hydronium ion at equilibrium. This yields a very useful approximate formula, that the concentration of hydronium ion at equilibrium in a solution that contains only a weak acid in water is given by
\[ {\rm[H_3O^+] = \sqrt{(K_a)(C_{HA})}}\]
We can arrive at a nearly identical result relating the concentration of a weak base and Kb to the hydroxide ion concentration [OH-]. The chemical equation for a weak base, B, in water is
\[\rm{B(aq) + H_2O(l) \rightleftharpoons BH^+(aq) + OH^-(aq)}\]
The equilibrium constant is
\[{\rm K_b = {{[OH^-][BH^+]} \over {[B]}}}\]
Using an identical derivation, we arrive at the approximate result where CB is the concentration of the weak base.
\[ {\rm[OH^-] = \sqrt{(K_b)(C_B)}}\]
When do these approximations fail? This question is difficult to answer, since it all depends on how how accurate an answer you are trying to find. They are always approximate. However, the approximation is better or worse in certain limits. This can be understood by examining what is being assumed in the approximation. There are two key assumptions. First, it is assumed that the initial concentration of hydronium ion (hydroxide ion) in pure water is zero. This is wrong. The concentration in pure water is very very small, but it is not zero. As a result, this approximation will fail when the solution is very dilute or the acid (base) is so weak that it essentially does not dissociate. The second assumption is that the amount of the acid that dissociates is so small that the concentration is essentially unchanged [ CHA - x ≈ = CHA ] When is this a reasonable approximation? When CHA >> x. This will be the case when the solution is concentrated and the acid is weak. Thus this approximation is better for concentrated solutions of weak acids with small Ka's.
Concept Question
In the following reaction, the equilibrium constant is 1.8 x 10-5?
\[ {\rm NH_3(aq) + H_2O(l) \leftrightarrow NH_4^+(aq) + OH^-(aq)}\]
If the initial concentrationof NH3 was 1M, what is the approximate concentration of NH3 at equilibrium?
(touch choices to toggle feedback on/off)
Acid (Base) Strength
We rank the relative strength of different acids in terms of the magnitude of their acid equilibrium constant Ka. The larger the Ka, the greater the hydronium ion concentration for a solution of that acid. If we compare a series of different acid solutions with the same concentration, the solution made from the acid with the largest Ka has the greatest hydronium ion concentration. We would refer to this acid as the "strongest" as it dissociates the most.
Typically this comparison is made only for "weak" acids. Strong acids have Ka's that are so large they are rarely tabulated (or utilized). It is generally assumed that strong acid Ka's are essentially infinitely large.
In contrast, weak acids do not ionize 100%. Take this table of a few weak acids that have been sorted by the Ka values.
| Acid Name | Formula | Ka |
| Hydrofluoric | HF | 3.5 x 10-4 |
| Formic | HCOOH | 1.8 x 10-4 |
| Acetic | CH3COOH | 1.8 x 10-5 |
| Hypochlorous | HClO | 3.0 x 10-8 |
| Hydrocyanic | HCN | 4.9 x 10-10 |
Of these acids the strongest is HF as it has the largest Ka. Hydrocyanic is the weakest of the group as it has the smallest Ka. This means that HF will dissociate to a greater extent when placed in water than the other acids. HCN will dissociate the least.
An analogous comparison can be made for weak bases looking at Kb. The largest Kb will protonate to the greatest extent in water and form the highest concentration of the hydroxide ions. The smallest Kb will form the lowest concentration of hydroxide in water.
Finally, a safety note. Weak acids/bases are not "weak" in the sense that they are not reactive. Weak acids and bases can be extremely dangerous especially in high concentrations. Don't let the name fool you. It is merely a means to rank how much they react in water to form hydronium or hydroxide ions. The stronger the acid, the larger the Ka, the more H3O+. The stronger the base, the larger the Kb, the more OH-.
Concept Question
Which of the following is the strongest acid? (touch choices to toggle feedback on/off)
© 2013 mccord/vandenbout/labrake