The Brønsted-Lowry definition of acids and bases is that:
An acid is a PROTON donor
A base is a PROTON acceptor.
A PROTON is an "H+" which is a hydrogen without an electron. The H+ swaps places between two different molecules (the donor acid and the acceptor base).
This idea that the proton is swapping place is a powerful way to view chemistry. Acid/Base compounds can be found in one of two forms. Either with the proton on the molecules, "the protonated form". Or with the proton off the molecule, "the de-protonated" form". The protonated form of the molecule is the acid form. The deprotonated form is the base form. Therefore each compound can be both an acid and a base depending on whether it is protonated or not. Such protonated/de-protonated pairs are known as conjugated pairs. It is important to be able to recognize such pairs as they can interchange simply by the adding or subtracting a proton. Whether we typically refer to a compound as an acid or base depends a great deal on the relative acid/base strength of the compounds (as will be seen later). But it is critical to realize that all acid base compounds are conjugate pairs from the Bronsted-Lowry perspective.
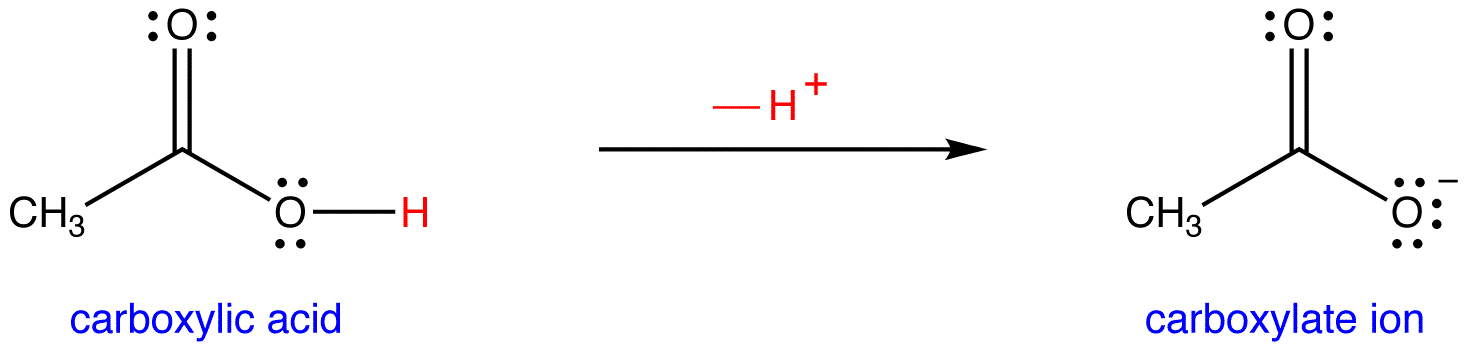
For example, a simple acid is acetic acid CH3COOH
Acetic acid can be either "protonated" as shown on the left or it can lose a proton and be "de-protonated" as is shown on the right. The de-protonated form is called the acetate ion. We will deal with naming compounds more later. For now, you should simply be able to identify that these two compounds are conjugate pairs as they differ by only one proton. The acetate ion is the conjugate base of acetic acid. Acetic acid is the conjugate acid of the acetate ion.
If we put acetic acid in water, some of the acid will donate a proton to water to form the hydronium ion, H3O+. In this case, acetic acid is the acid as it is donating the proton. Water is the base as it is accepting the proton. The acid converts to its conjugate base, the acetate ion. The base converts to its conjugate acid, the hydronium ion.
CH3COOH(aq) + H2O(l) ⇌ CH3COO- (aq) + H3O+(aq)
Every acid base reaction will have a reactant that is an acid and a reactant that is a base. This is what defines acid/base chemistry. In the Bronsted-Lowry defnition, these two will be exchanging a proton to generate their conjugate partners.
A video illustrating the definition of Bronstead-Lowry Acids and Bases. Examples are given of each type.
In the following reaction, which compound is the proton donor (for the forward reaction)?
\( {\rm NH_3(aq) + H_2O(l) \rightleftharpoons NH_4^+(aq) + OH^-(aq)}\)
(touch choices to toggle feedback on/off)
© 2013 mccord/vandenbout/labrake