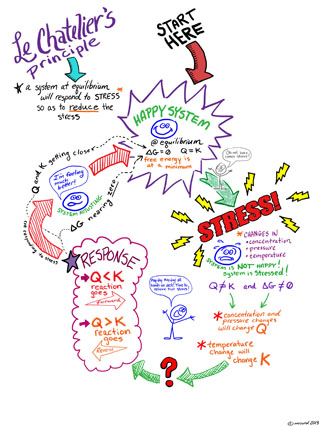
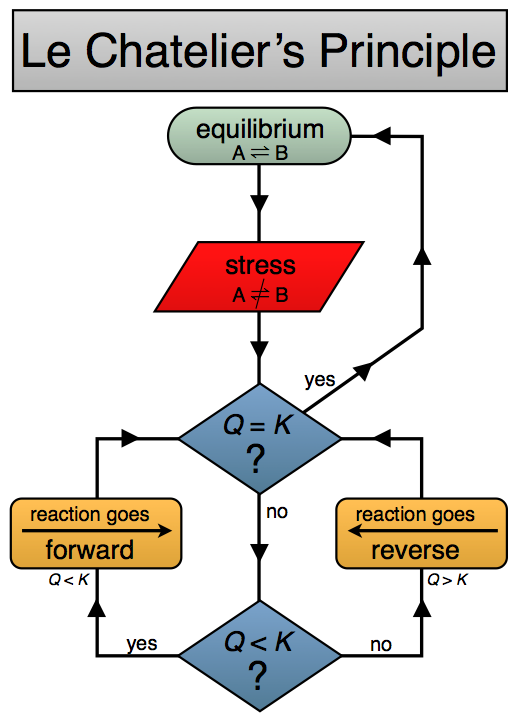
Le Chatelier's Principle is an idea about how a reaction mixture that is at equilibrium will react when it is perturbed away from equilibrium. This is a general idea that can help us to quickly have some insight into chemical equilibria and change.
Le Chatelier's Principle states
If a chemical system at equilibrium experiences a change, then the equilibrium shifts to counteract the imposed change and a new equilibrium is established.
By "change" we are moving the system away from equilibrium such that Q ≠ K. We can understand the direct the system need to move to re-establish equilibrium by thinking about what happens to Q when we "stress" or "change" the system.
We will deal with three particular examples.
Here are a couple of Le Chatelier's Principle diagrams for you to use and help you "visualize" the concept. Click on the images below to see a bigger version. One is a doodled thing with comments, the other a flow chart.
© 2013 mccord/vandenbout/labrake