Just as the protonation state can be determined for a conjugate acid/base pair by comparing the pH to the pKa, the protonation state for each proton in a polyprotic acid can be found making the same comparision.
That is, if you find the compound at a particular pH, how many of the protons are "on" the molecule (in the protonated form) and how many are "off" the molecule (in the deprotonated form)?
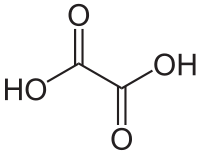
For example, consider the polyprotic acid, oxalic acid
This has two carboxylic acid groups (COOH). This compound could be written as (COOH)2. As a consequence, it has two acidic protons. The pKa for these two are
pKa1 = 1.25
pKa2 = 4.14
Examining the compound in aqueous solutions of different pH, it is possible to determine whether 1 or 2 protons would be "on" or "off" the molecule under different conditions.
First imagine the compound is in a solution that has a pH = 10. That is rather basic. The question is, "is pH=10 basic compared to the pKa's of the protons". This can be determined by comparing the pH and pKa's. In this case the pH is more basic than both the pKa's (pH > pKa). Therefore both protons will "deprotonated". The solution would have the majority of the compound in the fully deprotonated form (COO-)2. If instead the solution was very acidic situation pH = 0, then comparing the pH to the pKa's, the solution is more acidic than both the pKa's (pH < pKa) and both protons would be "on" the molecule. The predominate form in solution would be the fully protonated (COOH)2. Finally, consider a pH that is between the two pKa's. At a pH = 2.5, one proton would be "off" since this is more basic than the first pKa (that proton would be deprotonated), but it is more acidic than the second pKa (that proton would be protonated). The predominate molecular form would be the singly protonated (COO)-(COOH).
What about other pH values? What if the pH was equal to pKa2? Then we would expect to find the first proton completely off (as the pH is more basic than the first pKa), but the second proton would be at a pH where we would find it half protonated and half deprotonated.
© 2013 mccord/vandenbout/labrake