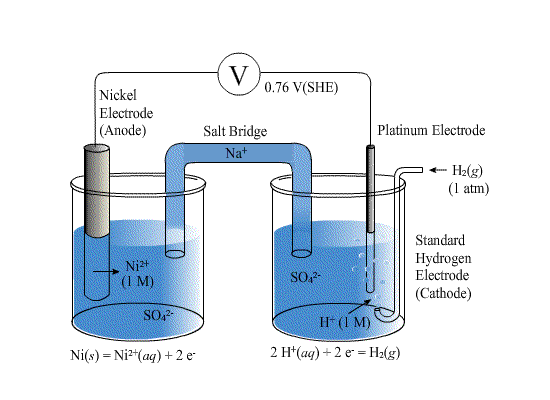
For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. The reaction we choose is the following.
\[\rm{2H^+(aq) + 2e^- \rightarrow H_2(g)}\]
Where the concentration of the H+ is 1M and the pressure of the H2 gas is 1 atm. Such an electrode is called a "standard hydrogen electrode" or SHE.
We can then make a cell with any other half reaction (under standard conditions where concentrations are 1M) and measure the potential (voltage).
For example here is a cell to compare the potential of the reaction of Ni to Ni2+ to the SHE.
We can then measure all such combinations and tabulate the data. Note: for some reactions, we are measuring the oxidation and for others the reduction, but we will always tabulate the reduction potential for the half reaction.