An electrochemical cell in which the chemistry is non-spontaneous is called a electrolytic cell. This means that the oxidation will not occur spontaneously at the anode and the reduction will not be spontaneous at the cathode. The chemistry we would like to occur is uphill in free energy. It can't happen unless we make it happen. For most chemical reactions, this can only be accomplished by coupling it to another reaction in which the free energy change is sufficiently negative that the two reactions together are spontaneous. In the case of electrochemistry, it is significantly easier as we can simply drive the reaction by attaching an external power supply.
The standard potential for an electrolytic cell is negative. The standard free energy for a reaction \(\Delta G^\circ \) is related to the standard potential, \(\mathcal{E}^\circ \), such that positive free energy (non-spontaneous) corresponds to negative potential. To make the reaction "go," we must apply a voltage that is significant enough to overcome the negative potential of the cell itself (it is spontaneous in the reverse direction).
Electrolytic cells thankfully don't have many names. However processes such as electroplating, or electrodeposition are typically electrolytic cells.
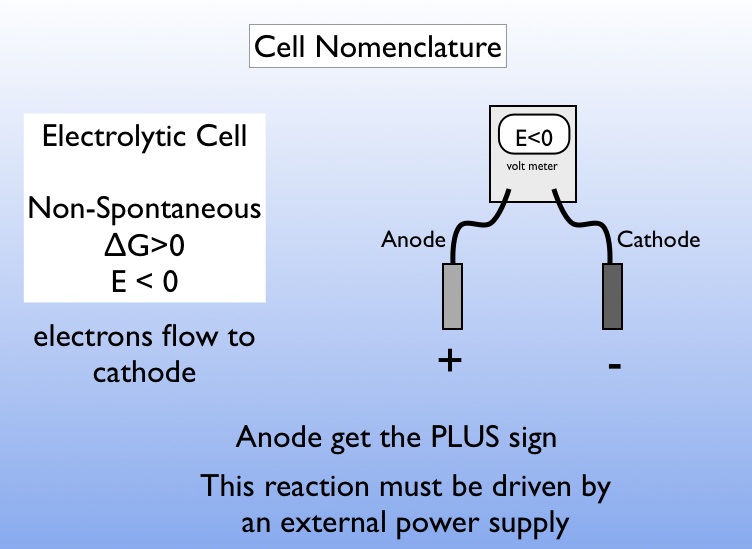
Below is a picture that summarizes the cell nomenclature for a electrolytic cell.
For an electrolytic cell \(\mathcal{E}^\circ \lt 0 \), \(\Delta G^\circ \gt 0\)
Electrons flow from anode to cathode (this is always the case). For an electrolytic cell however, this flow is not spontaneous but must be driven by an external power source.
In an electrolytic cell, the anode has the "+" sign.It is important to realize that an electrolytic cell and a voltaic cell as essentially exactly the same with the exception of what we "want to happen". The chemistry is always spontaneous in one direction. So if we chose a particular direction by calling something a "reactant" or "product" then it may or may not be spontaneous in that direction. Similarly by declaring one electrode the "anode" and one the "cathode" we are choosing where we would like the oxidation and reduction to occur. This may be spontaneous (a voltaic cell) or it may not be spontaneous (an electrolytic cell).
Generally electrolytic cells are used to generate unstable (high free energy) compounds or elements from stable (low free energy) compounds. With electrical work we can force the chemistry from low free energy back up to higher free energy. This can be extremely useful. We can use electricity to generate hydrogen and oxygen (high free energy) from water (low free energy). Or we can use electricity to generate sodium metal and chlorine gas from sodium chloride salt. This can lead to a slightly different physical configuration for an electrolytic cell. In a voltaic cell we need to separate the starting materials (reactants) from one another so that they do not spontaneously react with each other. For many electrolytic cells this is not necessary since the "reactant" is a stable compound like NaCl. However, it is critical for such an electrolytic cell that we separate the products from one another as they will spontaneously recombine. For example, in an electrolytic cell that produces Na(s) and Cl2(g) we start with NaCl(s). To make the cell first the NaCl is melted. Then the anode and cathode are placed into the molten NaCl. At the anode the Cl-1 is oxidized to Cl2 gas and at the cathode the Na+ is reduced to solid Na. These two elements will react with each other spontaneously to reproduce NaCl if they come in physical contact. Therefore, the cell needs to be constructed such that this never happens. Finally, such a cell does not require a salt bridge. This is because the molten NaCl is an ion conductor, and the reaction is removing anions at the anode and cations at the cathode so the charge balance is maintained.
© 2013 mccord/vandenbout/labrake