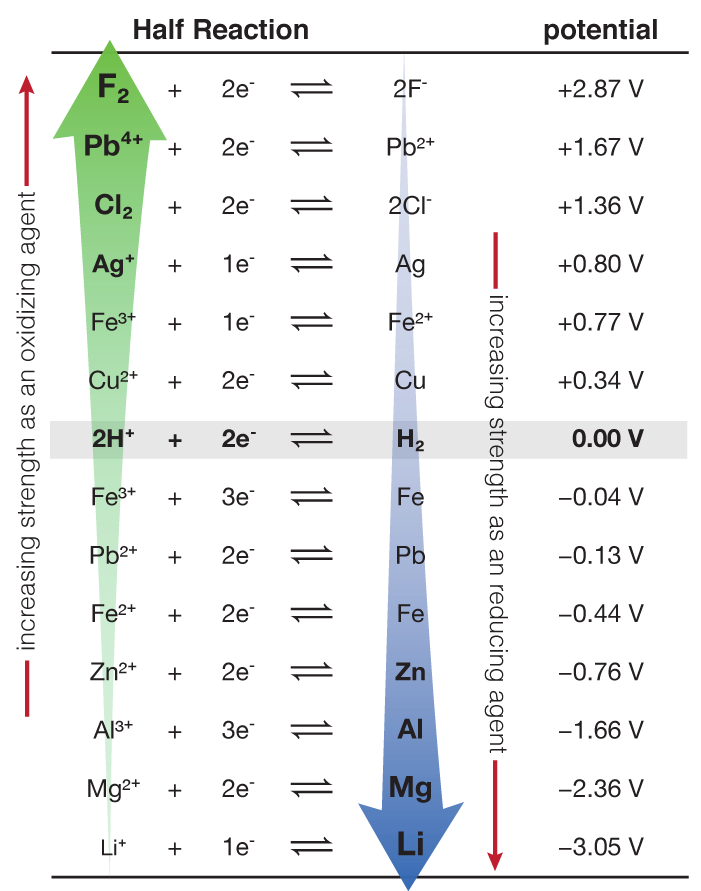
Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction potentials for all half-reactions, (or oxidation potentials but we need to pick one and stick to it).
Below is an abbreviated table showing several half-reactions and their associated standard potentials. All "standard potentials" are reduction potentials unless told otherwise.
Oxidizing Agents: At the top left of the table (where the green arrow is pointing) are the substances that are easiest to reduce. A better statement would be that those substances are ones that "want desperately" to be reduced, so much so that they will "forcefully" withdraw electrons from other species so that they can be reduced. This is the very definition of a good oxidizing agent. Fluorine gas is one of the best oxidizing agents there are and it is at the top of the table with the biggest most positive standard potential (+2.87 V).
Reducing Agents: At the other end, are reactions with negative standard potentials. This means that the desired path of the reaction is actually the reverse reaction. On the right side (product side) are substances that "want desperately" to lose their electrons and undergo an oxidation. These substances (ruled unsurprisingly by the alkali metals) will "force" their unwanted electrons upon other species. In doing so they become the definition of a powerful reducing agent. So the best reducing agents are at the bottom of the table on the right side and have the most negative standard potentials.
When looking at the table, we need to be careful since everything is written as a reduction. For example, from this table we can find the substance that is easiest to reduce. That is at the the top of the table (the F2/2F- redox couple). ALL the substances on the left are being reduced but the reactions become less and less likely as the potential goes from positive to negative. Contrary to this are the substances that are being oxidized. ALL the species being oxidized are on the right side of the table (a product). Li(s) is obviously the easiest to oxidize because it is the extreme case of this situation.
Look on the LEFT side of the half-reactions for substances that are going to be reduced. Look on the RIGHT side to find substances that are going to be oxidized.
Since Li is easy to oxidize, it is an excellent reducing agent (it reduces something else when it is oxidized). F2 is a great oxidizing agent (it oxidizes something else when it is reduced).
From this table, we can now figure out what reactions will be spontaneous. For example, if something is higher in the table (higher standard potential) it will run in the forward direction and the active reactant will be reduced. The reactions that are lower on the table (more negative standard potentials) will tend to run in reverse (right to left) and the reaction will be an oxidation where the active species on the right (aka: the product) is being oxidized.
Will Ag+ oxidize Fe? Yes. How do we know? The reduction potential for Ag+ is more positive than that for Fe2+. So Ag+ is a strong enough oxidizing agent to oxidize Fe (look for it on the RIGHT side) to Fe2+. On the other hand it could not oxidize chloride ions, Cl-, to chlorine gas, Cl2. Why? Because chlorine gas is a stronger oxidizing agent than silver ion.
Below is an image of our eBook's more extensive table of standard reduction potentials. You can find an even larger data set via the wikipedia link below the image.
Here is a Link to our eBook's Standard Reduction Table.
© 2013 mccord/vandenbout/labrake