An electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. This means that the oxidation will occur spontaneously at the anode and the reduction spontaneously at the cathode. We should note that the notation of something as a voltaic cell is a choice. Just as we choose what we want to call the "reactants" and "products" in a chemical reaction, our choice of anode and cathode is based upon what chemistry we would like to see occur. For a voltaic cell our choice ends up being the spontaneous choice. Below is an animated diagram of a voltaic cell showing electrons leaving the anode (on the left for oxidation), going through the wire, maybe a voltmeter, and then entering the cathode (on the right for reduction). Remember that in a voltaic cell, the spontaneous chemical reaction (redox rxn) is "pushing" and "pulling" those electrons through the circuit.
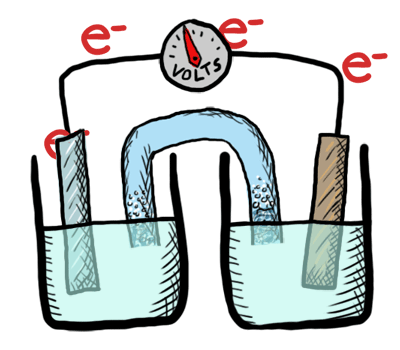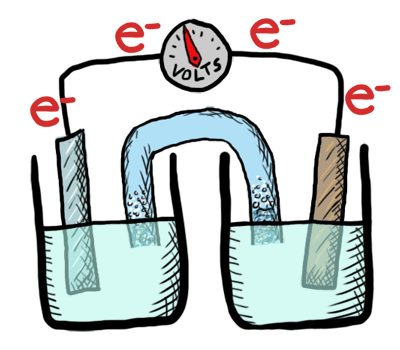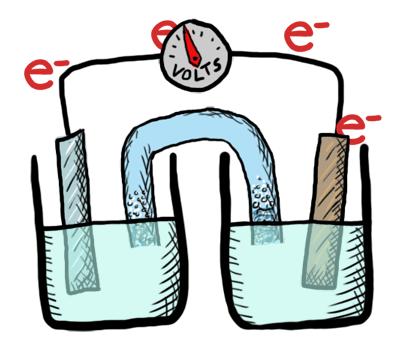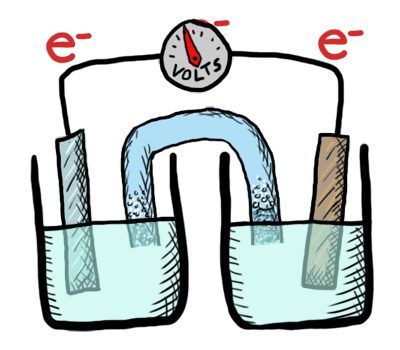
The standard potential for a voltaic cell is positive. The standard free energy for a reaction \(\Delta G^\circ \) is related to the standard potential, \(\mathcal{E}^\circ \), such that negative free energy (spontaneous) corresponds to positive potential. The beauty of electrochemistry and electrochemical cells is that we can now directly measure the free energy difference by measuring electrical potential.
Voltaic cells can be referred to as Galvanic Cells. This is a different word for the identical concept. They are also batteries. Since a battery is an electrochemical cell that produces a voltage (and current) spontaneously, it is a voltaic cell. You will find these three terms used interchangeably.
Below is a picture that summarize the ideas for a voltaic cell.
For a voltaic cell \(\mathcal{E} \gt 0 \), \(\Delta G \lt 0 \). This is the sign convention for spontaneous change.
Electrons always flow from the anode to the cathode in every electrochemical cell (voltaic and electrolytic) by the very definition of what occurs at the electrode surfaces. However, in voltaic cells the process is spontaneous - the electrons are driven through the external circuit by the free energy of the spontaneous redox reactions occurring in each half cell.
The one odd detail is the "sign" of the electrodes. For a voltaic cell the cathode is assigned the "+" sign. Why? Because it is actually drawing in electrons to "feed" the reduction going on. This means the electrode is inherently positive. Also, in this context, cations are migrating towards the cathode in the solution.
In an analogous way, the anode is pushing electrons out and away. Only a negative charge would spontaneously do that, so the anode on a voltaic cell is negative (-).
The "odd" part is that the signs on the cathode and anode in an electrolytic cell are the opposite way, the cathode is negative (-), and the anode is positive (+). This is because electrolytic cells are NOT spontaneous at all, there is often nothing there to push or pull electrons in either direction, like in a salt water solution (say aqueous Na2SO4). Electrons are FORCED upon the electrode that then becomes a cathode. Forcing electrons on an electrode will make it negative (-). Forcefully REMOVING electrons from the other electrode makes it positive and the anode (+). So electrolytic cells have the opposite signs associated with the cathode and anode as they do in voltaic cells.
© 2013 mccord/vandenbout/labrake