A catalyst is a substance that speeds up the rate of a chemical reaction but is not consumed during the course of the reaction. A catalyst will appear in the steps of a reaction mechanism, but it will not appear in the overall chemical reaction (as it is not a reactant or product). Generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. By lowering the activation energy, the rate constant is greatly increased (at the same temperature) relative to the uncatalyzed reaction.
There are many types of catalysts in the world. Many reactions are catalyzed at the surface of metals. In biochemistry, enormous numbers of reactions are catalyzed by enzymes. Catalysts can either be in the same phase as the chemical reactants or in a distinct phase.
Catalysts in the same phase are called homogeneous catalysts, while those in different phases are called heterogeneous catalysts.
For example, if we have Pt metal as a catalyst for the reaction of hydrogen gas and ethene gas, then the Pt is a heterogeneous catalyst. However, an enzyme in solution catalyzing a solution phase biochemical reaction is a homogeneous catalyst.
Another important idea about catalysts is that they are selective. That is the catalyst doesn't just speed up all reactions, but only a very particular reaction. This is the key to many chemical transformations. When you only want to perform a particular chemical change, you look for a catalyst that will speed up that specific reaction but not others. Enzymes are remarkable in this way. Living biological systems require a myriad of specific chemical transformations and there is a unique enzyme to catalyze each of them.
Catalysts can either be in the same phase as the chemical reactants or in a distinct phase.
Catalysts in the same phase are called homogeneous catalysts, while those in different phases are called heterogeneous catalysts.
For example, if we have Pt metal as a catalyst for the reaction of hydrogen gas and ethene gas, then the Pt is a heterogeneous catalyst. However, an enzyme in solution catalyzing a solution phase biochemical reaction is a homogeneous catalyst.
The effect of a catalyst is that it lowers the activation energy for a reaction.
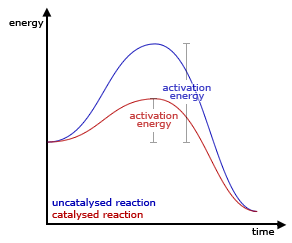
Generally, this happens because the catalyst changes the way the reaction happens (the mechanism). We can visualize this for a simple reaction coordinate in the following way.
In a more generally sense, the catalyzed reaction may have a number of new barriers and intermediates. However, the highest barrier will now be significantly lower than the previous largest barrier. For example, below is an example of the reaction path that shows a catalyzed and an uncatalyzed reaction. The path with the catalyst now has two steps along with an intermediate species. However, the barriers for both steps are much much lower than in the uncatalyzed reaction.
Many catalysts work in the same way. They provide a means for the reactant molecules to break bonds and then form temporary bonds with the catalyst. This means the catalyst must be somewhat reactive, but not too reactive (since we don't want these bonds to be permanent). For example, Pt metal serves as a catalyst for many reactions involving hydrogen gas or oxygen gas. This is because the Pt surface allows the H2 or O2 to break their bonds then form atomic species that are "bonded" to the Pt. However, these new bonds can be weak enough that the atomic species can then react with other molecules and leave the surface. In this way, the Pt metal returns to its pristine state after the reaction.
For example, the cartoon below depicts the reaction of ethene and hydrogen gas. The hydrogen lands on the surface and breaks its bond to form H atoms bonded to the surface (2). The double bond of the ethene is also broken and the two carbon atoms also bond to the surface (3). Then the H atoms can migrate until they collide with the bound carbon species and react (4) to form ethane which can then leave the surface (5).
Is this how all catalysts work? No. The possibilities for how a catalysts actually works are endless. Some catalysts actually change during the course of the chemical reaction, but then are returned to their original state at the end of the reaction. For example, MnO2 catalyzes the decomposition of H2O2 to water and oxygen gas by the following mechanism.
\[ \begin{array}{rclr} {\rm MnO_2(s) \; + \; H_2O_2(l) + 2H^+(aq)} \; & \rightarrow &\; {\rm Mn^{2+}(aq) \; + \; 2H_2O(l) \; + O_2(g)} & {\rm Step \;1 }\\ {\rm Mn^{2+}(aq) \; + \; 2H_2O_2(l)} \; & \rightarrow & \; {\rm Mn(OH)_2(aq) \; + 2H^+(aq) \; + \; O_2(g)} & {\rm Step \;2}\\ {\rm Mn(OH)_2(aq) \; + \; H_2O_2(l)} \; & \rightarrow & \; {\rm MnO_2(s) \; + 2H_2O(l)} & {\rm Step \; 3 }\end{array} \]
So in the net reaction there is no change in MnO2. However, during the reaction it is converted into Mn2+ as well as Mn(OH)2.A catalyst can be identified this way in a reaction mechanism as it appears in the "reactants" initially but then is reformed later in the reaction.
Catalysts can also function by "holding" molecules in particular configurations while simultaneously weakening some particular bonds. This allows the catalyst to essentially "help" the chemistry by arranging the reacts in favorable geoemetries as well as by weakening bonds that need to break along the reaction coordinate.
Enzymes are biological catalysts. They are proteins that fold into particular conformations such that they can help speed up very particular chemical reactions. For biochemical reactions, the reactant is typically called the substrate. The substrate is converted into the product. The mechanisms for many enzymes are very similar. The substrate(s) and the enzyme bind into a complex. The physical location on the enzyme in which the substrate binds is called the "active site". Once bound this complex can then weaken particular bonds in the substrate such that chemistry occurs to form the product. The product is weakly bound to the substrate such that it now dissociates and the enzyme is free to bind another substrate molecule.
The active sites in enzymes can be very specific such that the enzyme will only catalyze a very specific reaction for a very specific molecule. Typically there is an equilibrium between the bound complex and the free substrate and enzyme such that the binding could be reversible. In contrast, once the product is formed the backward reaction typically will never happen.
Substrate + Enzyme ↔ Complex → Product.
The activity of many enzymes can be blocked by molecules which mimic the substrate but don't do any chemistry. These molecules then effectively "turn off" the enzyme by blocking the active site and preventing binding of the substrate. Many pharmaceutical drugs operate in this way. Such molecules are typically called inhibitors as they inhibit the activity of the enzyme.