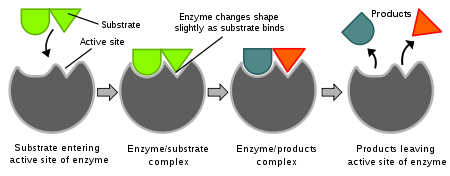
Enzymes are biological catalysts. They are proteins that fold into particular conformations such that they can help speed up very particular chemical reactions. For biochemical reactions, the reactant is typically called the substrate. The substrate is converted into the product. The mechanisms for many enzymes are very similar. The substrate(s) and the enzyme bind into a complex. The physical location on the enzyme in which the substrate binds is called the "active site". Once bound this complex can then weaken particular bonds in the substrate such that chemistry occurs to form the product. The product is weakly bound to the substrate such that it now dissociates and the enzyme is free to bind another substrate molecule.
The active sites in enzymes can be very specific such that the enzyme will only catalyze a very specific reaction for a very specific molecule. Typically there is an equilibrium between the bound complex and the free substrate and enzyme such that the binding could be reversible. In contrast, once the product is formed the backward reaction typically will never happen.
Substrate + Enzyme ↔ Complex → Product.
The activity of many enzymes can be blocked by molecules which mimic the substrate but don't do any chemistry. These molecules then effectively "turn off" the enzyme by blocking the active site and preventing binding of the substrate. Many pharmaceutical drugs operate in this way. Such molecules are typically called inhibitors as they inhibit the activity of the enzyme.
© 2013 mccord/vandenbout/labrake