A reaction coordinate is a path that links the reactant molecules and the products molecules. In many reactions, we can directly envision this coordinate as the length of a particular bond or bonds. In other cases, the reaction coordinate is used merely to represent some unknown coordinate. The key is that there are many many potential paths between reactants and products. The reaction coordinate represents the lowest energy path.
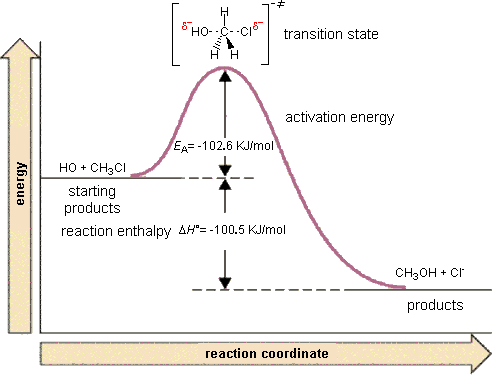
For example, in the reaction of CH3Cl + OH- to form CH3OH and Cl-, the mechanism of this reaction is a single step in which the CH3Cl collides with the OH- and forms the products. We can envision a reaction coordinate for this reaction which is the lengthening of the C-Cl bond (the one that is breaking) and the shortening of the C-O bond (the one that is forming).
Right in the middle of the breaking of the old bond and forming of the new bond, the system exists in what we call a "transition state" that is higher in energy than either the reactants or the products. Once it gets beyond this state, it proceeds along the reaction coordinate to form the products.
If a chemical mechanism has many steps, then it will have many hills and valleys along the reaction coordinate. Any minima that exist between the reactants and the products along the reaction coordinate are intermediates.
The reaction above has three steps (three barriers) and two intermediates. On the far left of the diagram are the reactant species and on the far right are the product species.
The transition state is the high energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state. As with the overall mechanism the rate of the reaction may only depend on the highest energy transition state as this will dominate the rate. Experimentally, the transition state is nearly impossible to observed or identify. This is because the molecules exist in this state for essentially zero time. Therefore, this is not a chemical species we can easily verify in the lab. However, from a theoretical point of view it is a critical concept. The configuration at the transition state is what is determining the rate. The initial and final states of the reaction essentially don't matter. What matters most is the configuration of the transition state. This is the point of maximum energy that the chemical species must pass through during the reaction. It is also important to realize that the reaction path we envision is the minimum energy path between the reactants and the products. There are other potential pathways but these require even more energy (have even higher energy transition states). Therefore the transition state of interest for a given reaction is the highest energy point along the lowest energy path.
© 2013 mccord/vandenbout/labrake