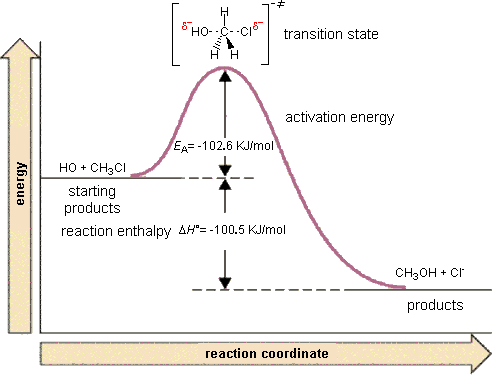
Activation Energy
The height of a barrier along the reaction pathway is the activation energy. The size of the activation energy depends on which way you approach the barrier as the energy on either side of the barrier could be higher or lower. Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol-1. Barriers are measured in energy per mole (typically kJ mol-1).