A major application of nuclear chemistry is the generation of electricity in nuclear power plants. First it is important to understand that in many ways nuclear power plants operate in much the same way as fossil fuel power plants. A reaction produces heat that is used to convert water into the steam that runs turbines that generate the electricity. Thus a nuclear power plant is simply a very sophisticated means to boil water.
A nuclear power plant uses nuclear chemistry to produce the heat. Specifically all nuclear power plants produce energy via fission reaction. Nearly all of them utilize the same fission reaction. This is the fission of U-235. One of these fission reactions is shown below.
\[{\rm ^{235}_{92}U \;\;+\;\; ^1_0n \;\;\rightarrow ^{141}_{56}Ba \;\;+ \;\;^{92}_{36}Kr\;\; + \;\;3 ^1_0n}\]
This is the fission of uranium-235 to make the fission products barium-141 and krypton-92 plus three neutrons. Note: there are neutrons on both sides of this reaction. It is important to shown them both in the reaction since the neutron instigates the reaction. The fission is actually of a uranium-236 nucleus that is created from the collision of a neutron and a uranium-235. While this reaction is a major one, the fission of U-236 results in a wide array of different fission products. This reaction is also extremely exothermic producing approximately 7 x 1013 J of energy per kilogram of U-235.
This fission reaction can be what is termed a chain reaction. Since one of the products, the neutron, is also a reactant, the reaction speeds up as it progresses. One neutron hits one U-235 and makes three neutrons. These go on to hit three U-235 that make 9 more neutrons. And so forth. More fission generates more neutrons that leads to more fission. A key to using fission for generating power is controlling this reaction.
In a power plant this is controlled by controlling the flux of neutrons. The uranium is typically converted into uranium oxide, UO2. Uranium oxide is chemically more stable than pure uranium metal allowing for less demand upon heating to high temperatures. It also cannot catch fire as it is already oxidized. The uranium oxide is then packed tightly into metal tubes called fuel rods. In a reactor the fuel rods are the source of the nuclear reaction and thermal energy. The reaction requires initiation by a neutron but U-235 also spontaneously decays emitting a neutron. In a nuclear power plant it is critical to control the reaction. This allows for regulation of the amount of power generated. It also prevents the reaction from running out of control. This is accomplished by placing "control rod" between the fuel rods. The control rods are "neutron poisons". They are composed of materials that readily absorb neutrons. Therefore when they are placed between the fuel rods they absorb the neutron from the fission reaction and stop the chain reaction. This is how the chemistry can be controlled to generate as much or as little energy as desired. The reaction is typically used in a large pool of water that absorbs the thermal energy and transports it to a heat exchanger to heat the water for the turbines (which in turn is cooled by another source of external water). This water also serves to "cool" the reactor. Without this water constantly removing the heat of the reaction, the fuel rods will get sufficiently hot that they will melt leading to a large unwanted radioactive mess. Additionally, if this happens the temperatures are reaching extreme conditions that lead to many other problems such as the generation of H2 and O2 gas from water. Not only does this produce large amount of high-pressure hot gas, this also happens to be an explosive mixture.
Another of the major challenges with nuclear power are the fission products that are created from the reaction. These include a wide array of radioactive isotopes. Some of these can be "recycled" and used as fission fuel, while other are simply "radioactive waste". These "spent fuel rods" emitting a large amount of radiation for a very long time. While not enough radiation to use them to generate power, they produce a great deal of heat. So much heat is constantly released that the spent fuel rods must be continuously cooled. If they are not cooled, they will meltdown leading to a hot radioactive chemically explosive mess. Thus a critical need for a nuclear plant is to have a constant flow of cooling water.
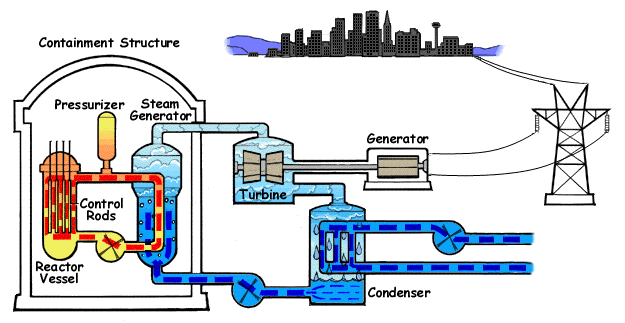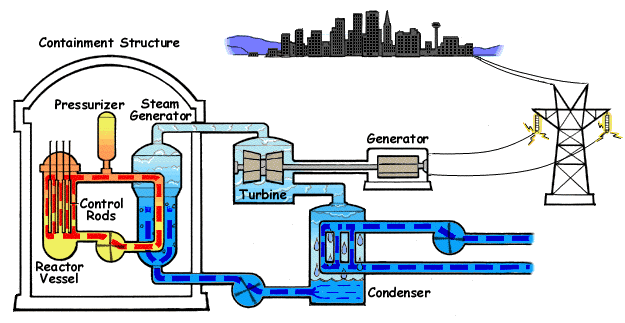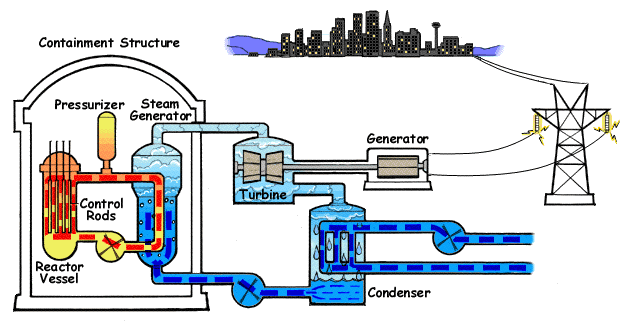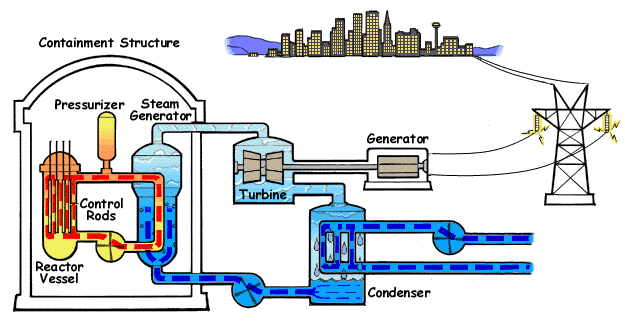
The picture above shows a schematic of nuclear power plant.
Vlog Brothers explain nuclear fission in light of the accident at the Fukushima nuclear power plant following the massive earthquake and tsunami in JapanFusion reactions are when two (or more) lighter nuclei come together to make a heavy nucleus in a process that is extremely exothermic. On an energy per kilo basis fusion is far and away the winner of biggest bang for the mass of fuel required. In addition, fusion can use hydrogen to make helium. This produces no long lived radioactive waste and is thus a much cleaner means to generate power. In addition, the fuel source is readily abundant. While there is little molecular hydrogen on earth, there is a virtually endless supply in other molecules such as water. While it would cost some energy to extract the hydrogen from water this chemical change would require orders of magnitude less energy than could be derived from fusion. However, currently fusion on earth is limited to short burst in large laboratories. Moreover, it currently takes more power to maintain the fusion reactions that is ever extracted. This is a common on the size of laboratory required. Although there are many interesting avenues being pursued in this regard by legions of physicist even as you read this. Currently fusion is limited to the Sun (and other stars).
Ionizing radiation is utilized in medicine as a means to treat cancer. This is called radiation treatment or radiotherapy. The idea is straight forward. In external radiation therapy, a beam of ionizing radiation is focussed onto a patient's cancerous tumors. The radiation ionizes molecules in the cancer cells leading to their demise. The downside to this treatment is that the ionizing radiation is also deadly for healthy cells. So the challenge is to destroy the cancer cells while minimizing the damage to the healthy cells. This is unfortunately difficult as the radiation must travel through the body to get to the cancerous cells.
Advances in radiation treatment has taken a number of forms. First, radiation sources can now be focussed and localized with much great effect. This limits the exposure to the desired areas to a greater extent. Nearly all radiotherapy treatments use high energy electrons as radiation. These electrons are generated utilizing high voltage in an electron accelerator (rather than utilizing electrons that result for radioactive decay). They can be focussed to tight beams with electric and magnetic fields. Another big advance in radiation therapy has been improved diagnostic imaging to locate the precise position of tumors within the body. Better imaging has allowed more limited treatment.
Finally, another form of radiation therapy is brachytherapy or internal radiation therapy. In brachytherapy a small piece of radioactive material is inserted into the body right next to the cancerous tumors. This can be done for a short period of time like a few minutes or a longer time of 24 hours during a hospital stay. The duration of the treatment is determined by the size of the tumor and the dosage of the radiation. The radioactive material can also be placed into the patient permanently. In the case of permanent implantation the patient is walking around with radioactive bits inside them. The radioactivity needs to be kept to a low dosage so that the patient does not expose the people around them to dangerous levels of radiation. Overtime the radiation levels drop to a point where the treatment has essentially stopped.
For example, Internal radiation treatment is an option for treating prostate cancer. This treatment generally uses small rods or "seeds" of a metal like palladium that includes a small amount of radioactive material. The palladium seed could include some palladium-103 (palladium has a large number of stable and unstable isotopes). 103Pd decays by electron capture to 103Rh while emitting 21keV gamma rays.
\[{\rm ^{103}_{46}Pd\;\;+\;\;_{-1}^0e \;\;\rightarrow \;\;^{103}_{45}Rh\;\;+ \;\;\gamma}\]
This decay has a half-life of 17 days. Because the half-life is so short the material loses most of its radioactivity within a few months. You cannot store radioactive material with a short half-life very long on a shelf as it is will simply be gone by the time you go to utilize it. Therefore, the material must be generated by transmutation shortly before use. This is done by bombarding palladium-102 with a neutron source to generate the palladium-103.
\[{\rm ^{102}_{46}Pd \;\;+ ^1_0n \;\;\rightarrow \;\;^{103}_{46}Pd}\]
Once generated it will begin to decay so these materials need to be constantly tracked and monitored to ensure the proper radiation dose is delivered to the patient.
There are number of schemes to estimate the age of an object based on the composition the isotopes it contains of particular elements. The most common scheme is radiocarbon dating.
Radiocarbon dating can estimate the age of organic material (carbon containing) based on the ratio of two carbon isotopes, 12C and 14C. 12C is the stable and most abundant form of carbon. 14C has a very low abundance and is radioactive and is therefore constantly decaying. Because 14C is always decaying one might guess that there would be less and less of it on earth all the time. However, radiocarbon dating works since the ratio of 12C to 14C is essentially constant on the Earth. This is because the 14C is continuously regenerated in the upper atmosphere.
Cosmic rays (high energy radiation) in the upper atmosphere excite molecules in the atmosphere leading to generation of neutrons. A neutron can then interact with a nitrogen-14 atom to produce carbon-14 and a proton.
\[{\rm ^{14}_{7}N \;\;+\;\; ^1_0n \;\;\rightarrow \;\;^{14}_{6}C\;\;+ \;\;^{1}_{1}p}\]
The 14C subsequently decays back to 14N by beta decay with a half-life of 5730 years. But the constant flux of cosmic rays means that the amount of 14C is in atmosphere is essentially at equilibrium. This means that the amount of 14C in CO2 molecules on earth is essentially constant as well. As most living organic organisms are exchanging CO2 with the atmosphere the 12C to 14C in living organisms is constant. It is about 1012 times more 12C to 14C. The ratio is constant up until the moment the organism dies. When the organism ceases to exchange CO2 with the atmosphere, the carbon within the organism is fixed. As these organisms are not in the upper atmosphere they are not interacting with cosmic rays to generate more 14C. Overtime their ratio of 14C to 12C starts to decrease. It falls by a factor of two every 5730 years. Thus, radiocarbon dating can be used to determine the age of old wooden tools by determining essentially when the tree they were made from was cut down.
1. Formation of carbon-14
2. Decay of carbon-14
3. Equality is for living organisms, and the inequality is for dead organism in which the carbon-14 is decaying.
There are many details to the accuracy and effectiveness of radiocarbon dating that deal with the nuances of the static nature of the 12C to 14C ratio (it is not exactly static and varies in time and location). But details aside, the general idea holds. Living organisms exchange CO2 and thus have a constant ratio (like the atmosphere). Once they die, this exchange stops and the amount of 14C decays away with time.
Because radioactive materials decay and release ionizing radiation, these materials are easy to track and locate by detecting the resulting radiation. There are a number of techniques that take advantage of this property in both the laboratory and in medicine.
In the lab, radioactive labeling is a means to track a particular molecule. The molecule is modified such that it has a "label" or radioactive isotope bound to it. Now wherever the molecule goes the radiation goes. There are many applications for such labeling experiments. For example, a new drug that is being tested might be synthesized to include a very tiny fraction of radioactive isotopes. As these isotopes are very easy to detect, the metabolism of the drug can be followed in studies by tracking the amount of the radioactive isotope in the urine of a test patient.
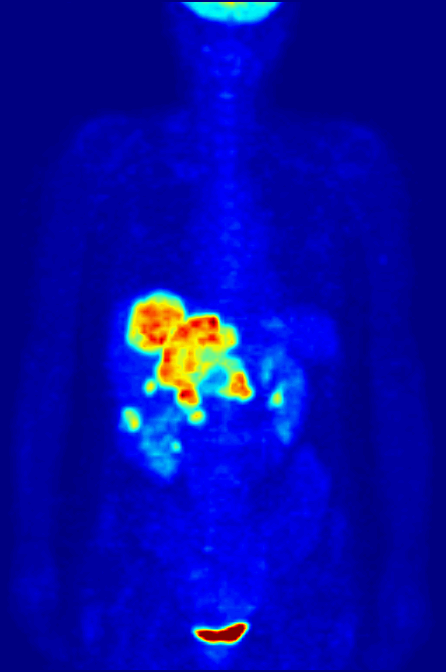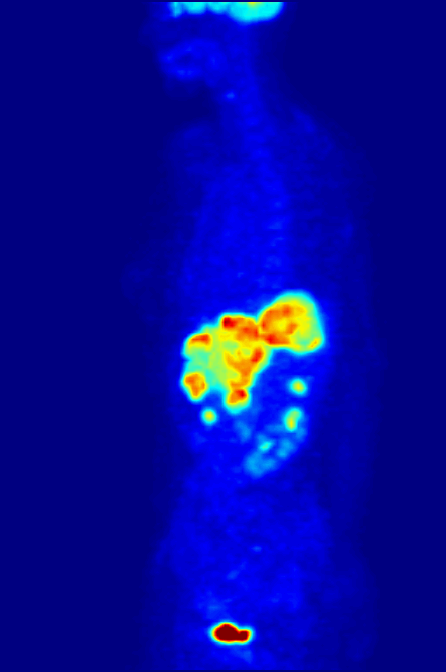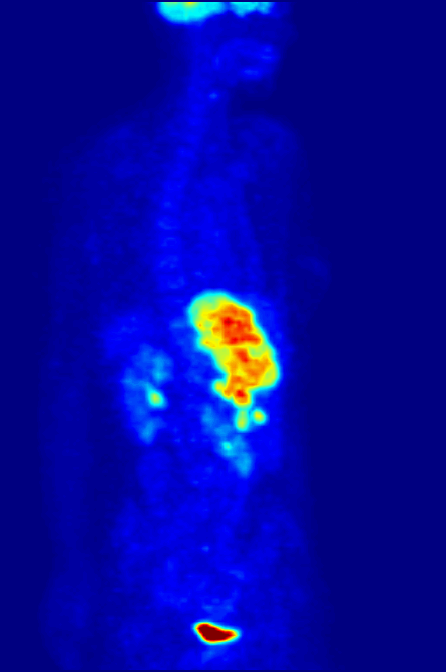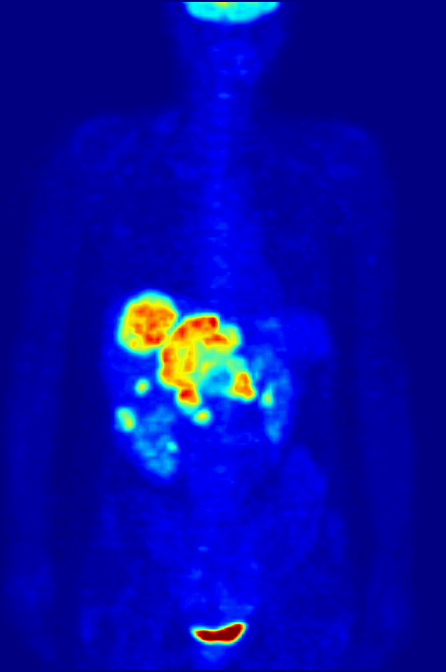
Another use of radioactive materials is in medical imaging. The decay of many radioactive isotopes results in the radiation that can be easily detected. A particularly powerful technique is positron emission tomography (PET). While the details of a PET scan are somewhat complex the general idea is straight forward. A patient ingests a compound with a small amount of radioactive isotope label. That isotope label decays by positron emission. The emitted positron then annhilates with and electron resulting in the emission to two gamma rays that travel in opposite direction. By detecting this pair of gamma ray photons the location of the labelled molecule can be located in three dimensional space! This allows for the generation of the 3-D image of the location of the labelled molecules within the patient. The most common use of PET scans is to locate cancerous tumors. A glucose analog is labelled with a radioisotope fluorine-18 that decays by positron emission. The cancerous cells take up the analog to a greater extent than the non-cancerous cells (as they have high glucose uptake). The PET scan can detect the location of the 18F and thus the location of the high glucose uptake cells.
Below is a PET scan image showing a whole body scan of an individual who had ingested the 18F-labelled glucose analog. As is normal in a PET scan the image reveals the high glucose uptake in the heart, bladder, kidneys and brain. Also visible are liver metastases of a colorectal tumor.(Image from wikicommons)
.
© 2013 mccord/vandenbout/labrake