A major application of nuclear chemistry is the generation of electricity in nuclear power plants. First it is important to understand that in many ways nuclear power plants operate in much the same way as fossil fuel power plants. A reaction produces heat that is used to convert water into the steam that runs turbines that generate the electricity. Thus a nuclear power plant is simply a very sophisticated means to boil water.
A nuclear power plant uses nuclear chemistry to produce the heat. Specifically all nuclear power plants produce energy via fission reaction. Nearly all of them utilize the same fission reaction. This is the fission of U-235. One of these fission reactions is shown below.
\[{\rm ^{235}_{92}U \;\;+\;\; ^1_0n \;\;\rightarrow ^{141}_{56}Ba \;\;+ \;\;^{92}_{36}Kr\;\; + \;\;3 ^1_0n}\]
This is the fission of uranium-235 to make the fission products barium-141 and krypton-92 plus three neutrons. Note: there are neutrons on both sides of this reaction. It is important to shown them both in the reaction since the neutron instigates the reaction. The fission is actually of a uranium-236 nucleus that is created from the collision of a neutron and a uranium-235. While this reaction is a major one, the fission of U-236 results in a wide array of different fission products. This reaction is also extremely exothermic producing approximately 7 x 1013 J of energy per kilogram of U-235.
This fission reaction can be what is termed a chain reaction. Since one of the products, the neutron, is also a reactant, the reaction speeds up as it progresses. One neutron hits one U-235 and makes three neutrons. These go on to hit three U-235 that make 9 more neutrons. And so forth. More fission generates more neutrons that leads to more fission. A key to using fission for generating power is controlling this reaction.
In a power plant this is controlled by controlling the flux of neutrons. The uranium is typically converted into uranium oxide, UO2. Uranium oxide is chemically more stable than pure uranium metal allowing for less demand upon heating to high temperatures. It also cannot catch fire as it is already oxidized. The uranium oxide is then packed tightly into metal tubes called fuel rods. In a reactor the fuel rods are the source of the nuclear reaction and thermal energy. The reaction requires initiation by a neutron but U-235 also spontaneously decays emitting a neutron. In a nuclear power plant it is critical to control the reaction. This allows for regulation of the amount of power generated. It also prevents the reaction from running out of control. This is accomplished by placing "control rod" between the fuel rods. The control rods are "neutron poisons". They are composed of materials that readily absorb neutrons. Therefore when they are placed between the fuel rods they absorb the neutron from the fission reaction and stop the chain reaction. This is how the chemistry can be controlled to generate as much or as little energy as desired. The reaction is typically used in a large pool of water that absorbs the thermal energy and transports it to a heat exchanger to heat the water for the turbines (which in turn is cooled by another source of external water). This water also serves to "cool" the reactor. Without this water constantly removing the heat of the reaction, the fuel rods will get sufficiently hot that they will melt leading to a large unwanted radioactive mess. Additionally, if this happens the temperatures are reaching extreme conditions that lead to many other problems such as the generation of H2 and O2 gas from water. Not only does this produce large amount of high-pressure hot gas, this also happens to be an explosive mixture.
Another of the major challenges with nuclear power are the fission products that are created from the reaction. These include a wide array of radioactive isotopes. Some of these can be "recycled" and used as fission fuel, while other are simply "radioactive waste". These "spent fuel rods" emitting a large amount of radiation for a very long time. While not enough radiation to use them to generate power, they produce a great deal of heat. So much heat is constantly released that the spent fuel rods must be continuously cooled. If they are not cooled, they will meltdown leading to a hot radioactive chemically explosive mess. Thus a critical need for a nuclear plant is to have a constant flow of cooling water.
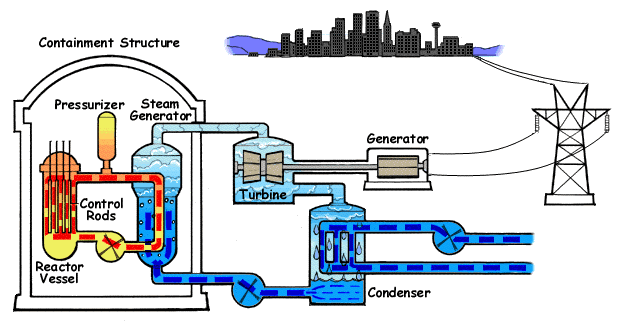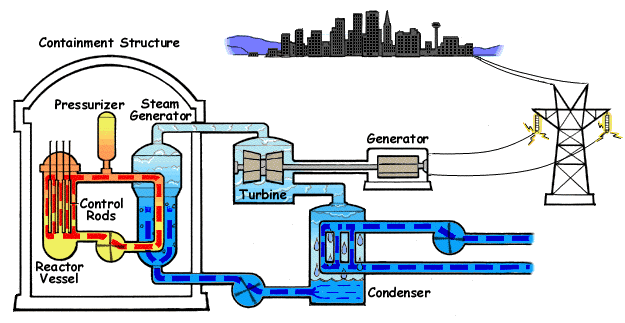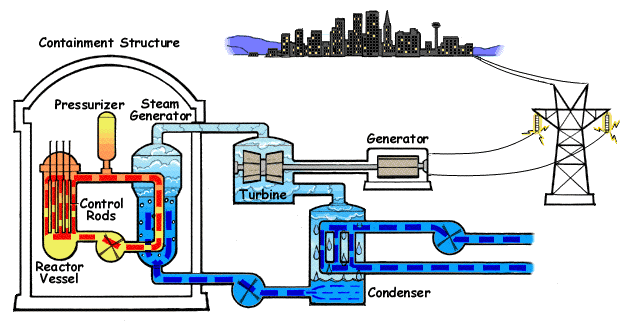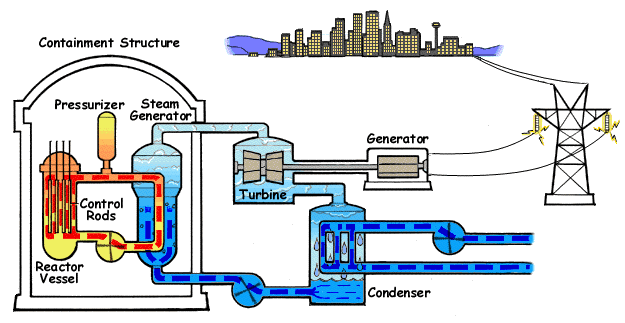
The picture above shows a schematic of nuclear power plant.
Vlog Brothers explain nuclear fission in light of the accident at the Fukushima nuclear power plant following the massive earthquake and tsunami in JapanFusion reactions are when two (or more) lighter nuclei come together to make a heavy nucleus in a process that is extremely exothermic. On an energy per kilo basis fusion is far and away the winner of biggest bang for the mass of fuel required. In addition, fusion can use hydrogen to make helium. This produces no long lived radioactive waste and is thus a much cleaner means to generate power. In addition, the fuel source is readily abundant. While there is little molecular hydrogen on earth, there is a virtually endless supply in other molecules such as water. While it would cost some energy to extract the hydrogen from water this chemical change would require orders of magnitude less energy than could be derived from fusion. However, currently fusion on earth is limited to short burst in large laboratories. Moreover, it currently takes more power to maintain the fusion reactions that is ever extracted. This is a common on the size of laboratory required. Although there are many interesting avenues being pursued in this regard by legions of physicist even as you read this. Currently fusion is limited to the Sun (and other stars).
© 2013 mccord/vandenbout/labrake