The half-life of a radioactive compound is the time it takes for half of the compound to decay. Because radioactive decay is a first order process (the number of nuclei that decays is proportional to the number of nuclei you have), the half-life is independent of the total number (or concentration).
Thus the half-life is a great description of a radioactive sample as it is independent of the amount of the sample.
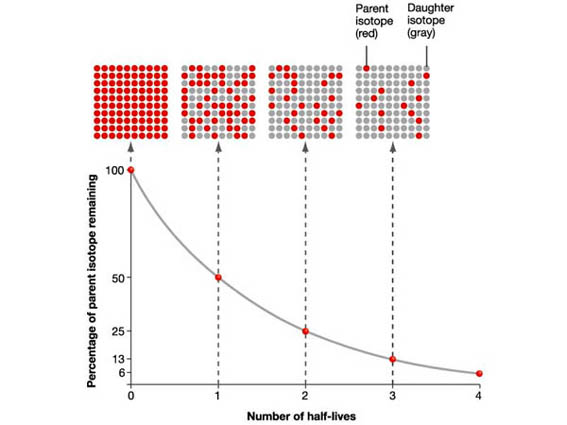
The graph depicts the number of radioactive isotope present as a function of time (looking at the population after several half lives). After one half-life, half of the sample has decayed. After another half-life, another half has decayed. So now there is only 25% of the original number of isotopes. After another half-life, half of the remaining isotope decays so you have only 12.5%....
The rate of decay of a nuclear compound follows what is called first order kinetics. The means that the rate of change is proportional the amount of the compound. This proportionality leads to an exponential decay of the amount of radioactive material. Thus over time the rate of radiation emitted from a radioactive compound decays exponentially.
Nuclear Decay Kinetics© 2013 mccord/vandenbout/labrake