A weak acid is a proton donor that when put in water will only partially dissociate.
A weak base is a proton acceptor that when put in water will only partially dissociate.
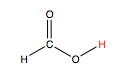
Let's look at the example of a weak acid, formic acid, HCOOH. This is a carboxylic acid that has the following structure. The acidic hydrogen is the one that is labelled in red. The electronegativity of the oxygens pull the electron density away from the hydrogen and leads to its partial ionization in solution.
Thus the equilibrium constant for this reaction
\[\rm{HCOOH(aq) + H_2O(l) \rightleftharpoons HCOO^-(aq) + H_3O^+(aq)}\]
is assumed to be small. We always write the equilibrium for a weak acid the same way. One mole of the acid plus one mole of water forms the hydronium ion (H3O+) and the deprotonated acid (the ion that is the acid minus the proton). We give this equilibrium constant a special name and call it Ka where the small a stands for acid (much like the sp in Ksp stood for solubility product). It is not different than any other equilibrium constant. It simply helps us to remember it is for this type of reaction written in this specific form. Thus the equilibrium constant will be
\[K_{\rm a} = {{\rm [H_3O^+][HCOO^-]} \over {\rm [HCOOH]}}\]
The "strength" of the weak acid will depend on the size of this equilibrium constant. The larger Ka is, the more it favors the dissociated side of the reaction. The smaller Ka, the less it favors the dissociated side of the reaction. For all weak acids, Ka is generally smaller than 1. That means for all weak acids the reactants are always favored. So if you put a weak acid in water the protonated form will have a higher concentration than the deprotonated form.
You'll also note that in this reaction the acid HCOOH is "changing" forms between having the proton HCOOH and not having the proton HCOO-. We can link these two species as what we could call "conjugate pairs". The protonated form HCOOH is the acid and the deprotonated form HCOO- is the conjugate base. That is formic acid (HCOOH) and the formate ion (HCOO-) are a conjugate pair. In the same reaction, the water is the base (deprotonated) while H3O+ (the protonated form) is the conjugate acid.

The base here is the deprotonated form of the acid
We can also write similar expressions for a base. Take ammonia. When it is placed in water it accepts a proton from water
\[\rm{NH_3(aq) + H_2O(l) \rightleftharpoons NH_4^+(aq) + OH^-(aq)}\]
Again NH3 is the base (deprotonated) and its conjugate acid is NH4+. The equilibrium constant for this reaction is written as Kb
\[K_{\rm b} = {\rm {[NH_4^+][OH^-]} \over {[NH_3]}}\]
the strength of the base will depend on the magnitude of Kb. The larger Kb the stronger the base.

The acid here is the protonated form of the base