A titration is an analytical chemistry technique that is often used to characterize an acid/base solution. In a titration, a strong acid/base of accurate concentration is added stepwise in small amounts (aliquots) to incrementally neutralize the solution. Titration are performed to either measure the concentration of an unknown solution and/or to determine the Ka (Kb) of an unknown acid (base).
During a titration you have two solutions: the analyte and the titrant. The analyte is the "unknown" solution for which you would like to know either the concentration or the equilibrium constant. The titrant is the "known" solution which has a precise and accurate concentration. The analyte can either be an acid or base and it can be either weak or strong. The titrant is generally a strong acid or base. Since the titration is a neutralization, acid analytes are titrated with strong bases. Basic analytes are titrated with strong acids. The titration is performed by slowly adding the titrant to the analyte solution in small amounts called aliquots. After each addition of an aliquot the pH of the solution is measured. This is performed until the solution has essentially experinced the entire range of pH conditions from acidic to basic (or basic to acidic).
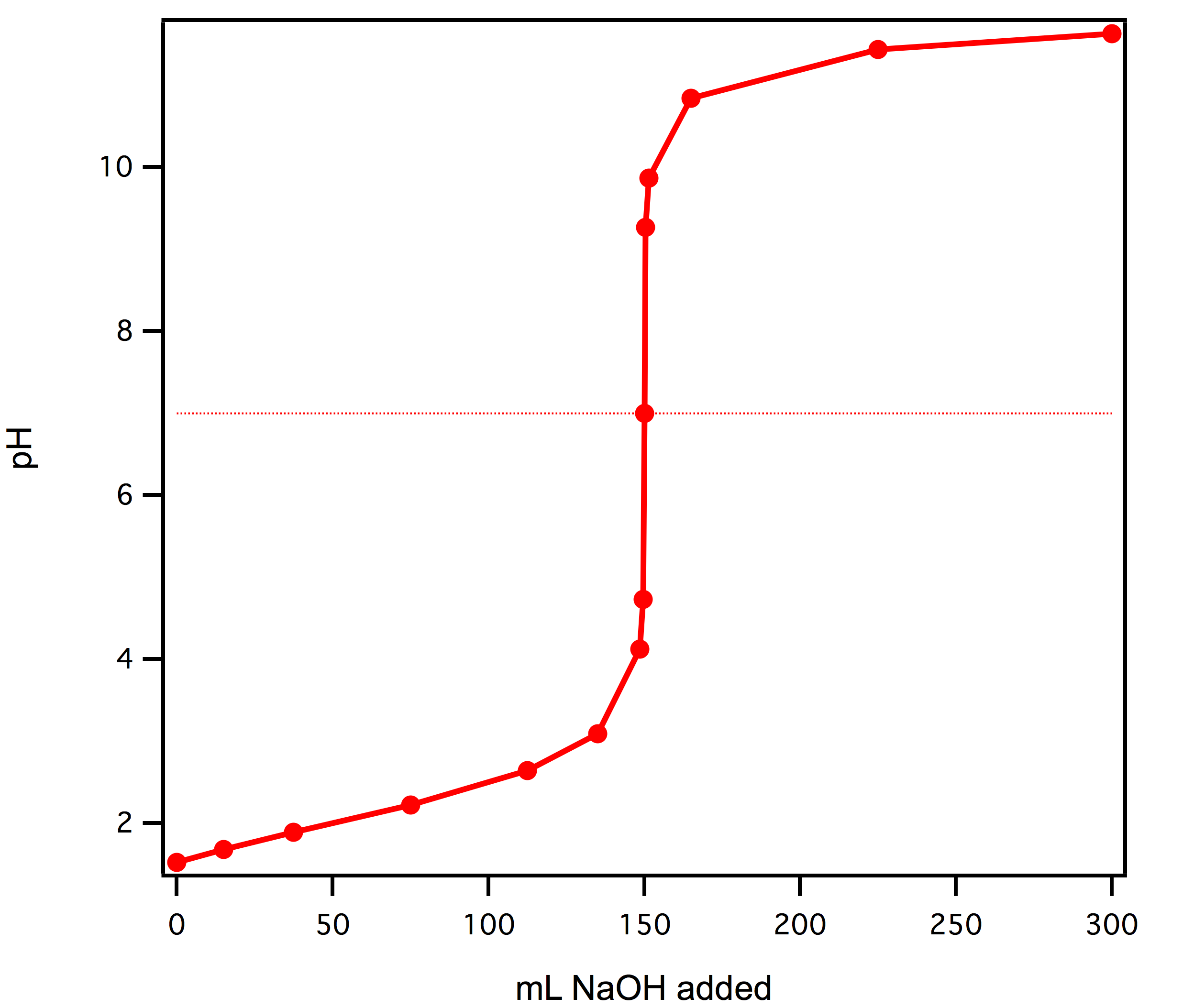
As an example, take a strong acid solution as an analyte that is titrated with a strong base. As the equilibrium constant of a strong acid is not of interest, the key to this titration is to accurately measure the concentration of the analyte solution. In this case, the pH of the analyte starts out very low (as the anlyte solution is a strong acid). As strong base is titrated into the solution, the pH increases slightly but in general will not change much. However, at some point the number of moles of base that have been added to the solution will be equal to the number of moles of acid in the original analyte. At this point the pH will change very dramatically as the solution will now be neutral. After this adding any more of the strong base will rapidly make the solution basic. It is the very large change in pH over a small range of the added titrate volume that is the reason to perform a titration. The point at which the number of moles of added base are equal to the number of moles of acid in the analyte solution is called the equivalence point. It is easy to identify this point in a titration because it is the volume at which the pH is rapidly changing. Technically, the equivalence point is where the titration curve exhibits an inflection point. At this point the curve has the steepest slope. The volume at the equivalence point can be used with the known concentration of the titrant to determine how many moles have been added to the solution. At the equivalence point the moles of added base will be equal to the moles of original acid, this allows the determination of the number of moles of original acid. This can then be combined with the original volume of the analyte solution to determine its concentration. In practice it is very important to use small aliquots to accurately determine the exact volume at the equivalence point.
The graph above shows the titration of a 50mL of a strong acid, HBr, of unknown concentration vs a volume of NaOH added. The concentration of the NaOH solution is known to be 0.1M. The equivalence point is at 150 mL. At the equivalence point 0.015 moles of OH- have been added to the solution (.15L x 0.1 M). This means that the original acid solution contained 0.015 moles of HBr. The concentration of the original solution must have be 0.3M (0.015 moles/0.05 L). (Note: for a strong acid and strong base titration the equivalence point is at a pH=7. This is because at this point you have equal moles of added base as acid in the original solution. Therefore at the equivalence point the solution has formed a neutral salt and the pH is 7).
Titrations of weak acids or bases can also be used to determine the Ka (Kb) of the analyte in addition to the concentration. For example, examine the the titration of an unknown acid with strong base. Initially the analyte has essentially all of the acid in its protonated form. It is simply a solution of a weak acid. As weak acid dissociates only slightly the majority of the compound is in the protonated form. As strong base is titrated into the solution this compound is converted from its protonated state to its deprotonated state. The titration takes the analtye from essentially fully protonated to fully depronated. Now at the equivalence point all of the anlayte will have been neutralized to form a basic salt. Therefore at the equivalence point the solution is essentially that of a weak base and the pH will be greater than 7. This point can again be utilized to calculate the concentration of the analyte solution in the exact same way as was done in the strong acid example above. However now additional information can be deduced from the half-equivalence point. The half equivalence point is when exactly half of the original analyte has been neutralized. This volume is easy to determine form the titration as it is half of the volume at the equivalence point. At this volume the concentration of the protonated form and deprotonated form of the acid are equal. This is a buffer solution. It is also a very special buffer. Since the two species have equal concentration this is the point at which for a weak acid the pH = pKa. Thus in the titration of a weak acid with a strong base the pKa of the acid can simply be read off the graph as the pH at the half-equivalence point (to the value of the Henderson-Hasselbach approximation).
The graph above shows the titration of 50 mL of a weak acid solution with 0.1 M NaOH. The equivalence point is clearly at 100 mL of added NaOH. Note that at the equivalence point the pH is basic (~8.8). Since the concentration of the NaOH is 0.1 M we can deduce that the concentration of the original acid solution must have been 0.2 M (equal moles of acid in the original 50 mL solution as in the 100 mL of added 0.1 M strong base). The Ka of the acid can also be determined. The half-equivalence point is the volume that is half the volume at the equivalence point. In this example that would be 50 mL. The pH at this point is 4.75. At this point the system should be a buffer where the pH = pKa. Thus the pKa of this acid is 4.75. The Ka is then 1.8 x 10-5 (10-4.75).
Titrations of weak bases with strong acids are very similar. Except that at the equivalence point all of the base will have been converted to its conjugate acid. The half-equivalence point will be a basic buffer with the pOH = pKb.
© 2013 mccord/vandenbout/labrake