In an electrochemical cell, we physically separate the oxidation and reduction chemistry in different "compartments". The electrons from the oxidation are then run through an external circuit before being used in the reduction reaction. As this is moving negative charge from one location to another, we need to compensate for this by moving other charges to balance this displacement of charge. This is accomplished by using a salt bridge that allows the migration of spectator ions to balance the flow of electrons.
Each half of the electrochemical cell has an electrode to which the wire for our external circuit is connected. The chemistry takes place at the surface of this electrode. The electrode on the oxidation side is called the anode. The electrode on the reduction side is called the cathode. As a short hand, we simply refer to the two sides of the cell as the anode side and the cathode side.
For any given set of conditions, the chemistry is spontaneous in only one direction. When the cell is set up such that this is the direction we desire, we call the cell a voltaic cell (or a galvanic cell or a battery). When we want the chemistry to go in the non-spontaneous direction, the cell is an electrolytic cell and requires an external power source to drive the chemistry in a non-spontaneous direction. It is important to note that this is one of the amazing things about electrochemistry. We can use an external voltage to change the direction of a chemical reaction by adjusting the free energy (we essentially get to chose which is lower in free energy the products or reactants). So with an electrochemical cell, we can get a chemical reaction to move in both the spontaneous and the non-spontaneous direction. You are all familiar with this concept but you might not have thought about it as chemistry. When you are using a battery you are running a voltaic electrochemical cell in the spontaneous direction. When it gets to equilibrium the voltage on the battery is zero and no more current flows. However, you can "recharge" the battery by attaching it to an external power supply and forcing the chemistry to go in the reverse direction until you have regenerated the original chemical contents.
In an electrochemical cell, we are running a redox reaction, but we have physically separated the oxidation and reduction reactions into different locations. These take place in different "halves" of the cell.
We then connect the two halves of the cell with an external wire to allow for electron flow from one side to the other. We also connect the two halves with a "salt bridge" to allow spectator ions to flow to counter balance the electron flow.
The oxidation and reduction reactions are now in different locations. To allow the electrons to flow from the oxidation reaction (that is producing electrons) to the reduction reaction (that is consuming the electrons), we need to connect the two reactions electrically. This is accomplished by using metal electrodes. These electrodes serve as the place where the chemistry is taking place. Sometimes they are part of the reaction. Sometimes they are simply used as a means to "deliver/collect" the electrons. The electrode in the oxidation reaction is called the anode. The electrode for the reduction reaction is called the cathode. The electrons flow from the anode to the cathode.
Let's look at a picture of the cell below which contains zinc metal and zinc ions on the oxidation side and copper metal and copper ions on the reduction side.
The left hand side is a picture of the anode half of the cell where the oxidation is taking place. The solid piece of zinc is the anode. The righthand side is the reduction half where the copper 2+ ions are being reduced to copper metal. The solid piece of copper is the cathode. The two sides are connected by a wire that allows electrons to flow from the anode to the cathode. They are also connected by a salt bridge. Since negative charge is flowing from the anode side to the cathode side, some spectator ions need to compensate this flow (or the cathode side will end up with lots of extra negative charge). So in the salt bridge either the positive sodium ions could flow from the anode side to the cathode side or the negative nitrate ions could flow from the cathode side to the anode side. Or both could happen.
For some reactions, neither the reactants nor the products are a metal. In this case, an inert (non-reactive) metal electrode is required to deliver/collect the electrons for the reaction.
For example, in the cell below at the anode, Fe2+ is oxidized to Fe3+. This reaction occurs at the surface of a Pt electrode. Also, note the ions in the salt bridge could be any strong electrolyte. They don't have to participate in the chemistry.
\[\rm{2Fe^{2+}(aq) \; + \; Cu^{2+}(aq) \rightarrow 2Fe^{3+}(aq) \; + Cu(s)\; }\]
It becomes very cumbersome to draw a picture of an electrochemical cell each time you would like to discuss one.
As such, we have developed a short hand notation for a cell.
For the cell above we have the anode on the left and the cathode on the right. The oxidation is occurring at the anode
\[\rm{Ni(s) \rightarrow Ni^{2+}(aq) + 2e^-}\]
The reduction is occurring at the cathode
\[\rm{2H^+(aq) + 2e^- \rightarrow H_2(g)}\]
The overall reaction is
\[\rm{Ni(s) + 2H^+(aq) \rightarrow Ni^{2+}(aq) + H_2(g)}\]
Rather than having this picture of the cell, we have a shorthand that is the same moving from left to right in our diagram. Starting with the anode on the far left, we write the atomic symbol for the solid material for which the anode is composed. Next we show the aqueous components in the anodic-half of the cell. We separate each component with a "|" symbol (vertical line). All aqueous components of the anodic half-reaction will be listed as part of the aqueous phase. Note that the order within the aqueous phase is not important, we just make sure that all necessary species are listed. Thus for our cell, we only have to list Ni2+ in the aqueous phase because there are no other species in the half-reaction. The anodic half-cell has Ni(s) being oxidized to Ni2+ in solution. Because Ni(s) itself is a conductor (a metal), we can just use it as the anode. In shorthand notation we would write the anode-half of the cell as
\[\rm{Ni\;\bigl|\;Ni^{2+}}\]
Next we would denote the cathode half of the cell. Again we would list all solution species followed by a vertical bar for phase change until we reach the anode material which will be listed on the far right of the notation. Here the reactant is H+, the product is H2, neither of which is a conductor which means an inert electrode must be used which will be Pt (the cathode). Even though our reduction reaction has two moles of H+ for each mole of H2 we do not show any stoichiometry in the shorthand notation - we simply list the reactive species that identifies the right half reaction. So the cathode-half of the cell shown would be\[\rm{ H^+ \;\bigl| \;H_2 \;\bigl| \;Pt}\]
Finally, we put the anode and the cathode sides together separated by the salt bridge. The salt bridge is shown as "||" (double vertical line). Thus the final cell diagram is
\[\rm{Ni\;\bigl|\;Ni^{2+}\;\; \bigl|\bigl|\;\; H^+ \;\bigl| \;H_2 \;\bigl| \;Pt}\]
This is the most generic of notations. To be more specific we might include the concentrations (or pressures) along with the phases.
\[\rm{Ni(s)\;\bigl|\;Ni^{2+} (aq,1M)\;\; \bigl|\bigl|\;\; H^+ (aq,1M) \;\bigl| \;H_2 (g, 1 atm) \;\bigl| \;Pt(s)}\]
Reading this notation we know that "on the left" is the implied anodic reaction which in this case is a nickel metal anode that is oxidizing to Ni2+ in solution. That solution is then connected by a salt bridge to a solution of H+ (acid) that are being reduced to H2 gas at a Pt cathode (the cathodic side, the right side). The left side of the cell shown is always to be written as an oxidation: Ni(s) -> Ni2+(aq) + 2e–. The right side of the cell shown is always to be written as a reduction: 2H+(aq) + 2e– -> H2(g). Also remember that in the shorthand cell notation no stoichiometry is shown at all - just species. You will still have to balance the two half reactions against each other in order to determine the overall cell reaction and how many moles of electrons are transferred per mole of reaction completed.
For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. The reaction we choose is the following.
\[\rm{2H^+(aq) + 2e^- \rightarrow H_2(g)}\]
Where the concentration of the H+ is 1M and the pressure of the H2 gas is 1 atm. Such an electrode is called a "standard hydrogen electrode" or SHE.
We can then make a cell with any other half reaction (under standard conditions where concentrations are 1M) and measure the potential (voltage).
For example here is a cell to compare the potential of the reaction of Ni to Ni2+ to the SHE.
We can then measure all such combinations and tabulate the data. Note: for some reactions, we are measuring the oxidation and for others the reduction, but we will always tabulate the reduction potential for the half reaction.
Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction potentials for all half-reactions, (or oxidation potentials but we need to pick one and stick to it).
Below is an abbreviated table showing several half-reactions and their associated standard potentials. All "standard potentials" are reduction potentials unless told otherwise.
Oxidizing Agents: At the top left of the table (where the green arrow is pointing) are the substances that are easiest to reduce. A better statement would be that those substances are ones that "want desperately" to be reduced, so much so that they will "forcefully" withdraw electrons from other species so that they can be reduced. This is the very definition of a good oxidizing agent. Fluorine gas is one of the best oxidizing agents there are and it is at the top of the table with the biggest most positive standard potential (+2.87 V).
Reducing Agents: At the other end, are reactions with negative standard potentials. This means that the desired path of the reaction is actually the reverse reaction. On the right side (product side) are substances that "want desperately" to lose their electrons and undergo an oxidation. These substances (ruled unsurprisingly by the alkali metals) will "force" their unwanted electrons upon other species. In doing so they become the definition of a powerful reducing agent. So the best reducing agents are at the bottom of the table on the right side and have the most negative standard potentials.
When looking at the table, we need to be careful since everything is written as a reduction. For example, from this table we can find the substance that is easiest to reduce. That is at the the top of the table (the F2/2F- redox couple). ALL the substances on the left are being reduced but the reactions become less and less likely as the potential goes from positive to negative. Contrary to this are the substances that are being oxidized. ALL the species being oxidized are on the right side of the table (a product). Li(s) is obviously the easiest to oxidize because it is the extreme case of this situation.
Look on the LEFT side of the half-reactions for substances that are going to be reduced. Look on the RIGHT side to find substances that are going to be oxidized.
Since Li is easy to oxidize, it is an excellent reducing agent (it reduces something else when it is oxidized). F2 is a great oxidizing agent (it oxidizes something else when it is reduced).
From this table, we can now figure out what reactions will be spontaneous. For example, if something is higher in the table (higher standard potential) it will run in the forward direction and the active reactant will be reduced. The reactions that are lower on the table (more negative standard potentials) will tend to run in reverse (right to left) and the reaction will be an oxidation where the active species on the right (aka: the product) is being oxidized.
Will Ag+ oxidize Fe? Yes. How do we know? The reduction potential for Ag+ is more positive than that for Fe2+. So Ag+ is a strong enough oxidizing agent to oxidize Fe (look for it on the RIGHT side) to Fe2+. On the other hand it could not oxidize chloride ions, Cl-, to chlorine gas, Cl2. Why? Because chlorine gas is a stronger oxidizing agent than silver ion.
Below is an image of our eBook's more extensive table of standard reduction potentials. You can find an even larger data set via the wikipedia link below the image.
Here is a Link to our eBook's Standard Reduction Table.
We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions.
For example, imagine we had the following cell.
\[\rm{Ni\;|\;Ni^{2+} \;\; || \;\; Fe^{3+} , \;\;Fe^{2+} \;| \;Pt}\]
We have at the anode (left) side the reaction
\[\rm{Ni \rightarrow Ni^{2+} + 2e^-}\]
and at the cathode (right) side the reaction
\[\rm{Fe^{3+} + 1 e^- \rightarrow Fe^{2+}}\]
The standard potential for this cell is simply
\[\rm{\mathcal{E}^{\circ} = \mathcal{E}^{\circ}_{cathode} - \mathcal{E}^{\circ}_{anode}}\]
It's even OK to be very literal about the two sides and you can get the standard cell potential with this equation as well
\[\rm{\mathcal{E}^{\circ} = \mathcal{E}^{\circ}_{right} - \mathcal{E}^{\circ}_{left}}\]
Where \(\mathcal{E}^{\circ}\) for each electrode is the standard reduction potential for the half-reaction. Using our table of standard reduction potentials we find for
\[\rm{Ni^{2+} + 2e^- \rightarrow Ni(s) \qquad \mathcal{E}^{\circ} = -0.25 V}\]
\[\rm{Fe^{3+} + e^- \rightarrow Fe^{2+} \qquad \mathcal{E}^{\circ} = +0.77 V}\]
So the standard potential is
\[\rm{\mathcal{E}^{\circ} = \mathcal{E}^{\circ}_{cathode} - \mathcal{E}^{\circ}_{anode} = 0.77 - (-0.25) = +1.02 V }\]
VERY IMPORTANT. Note: the potential is simply the energy difference between the two half reactions. Do NOT try to multiply the potentials by the number of electrons! The number of electrons simply relates how many electrons there are per reaction. How many Fe3+ will be reduced per Ni atom that is oxidized. The potential difference (free energy difference) between the two half-reactions is not dependent on the number of electrons.
An electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. This means that the oxidation will occur spontaneously at the anode and the reduction spontaneously at the cathode. We should note that the notation of something as a voltaic cell is a choice. Just as we choose what we want to call the "reactants" and "products" in a chemical reaction, our choice of anode and cathode is based upon what chemistry we would like to see occur. For a voltaic cell our choice ends up being the spontaneous choice. Below is an animated diagram of a voltaic cell showing electrons leaving the anode (on the left for oxidation), going through the wire, maybe a voltmeter, and then entering the cathode (on the right for reduction). Remember that in a voltaic cell, the spontaneous chemical reaction (redox rxn) is "pushing" and "pulling" those electrons through the circuit.
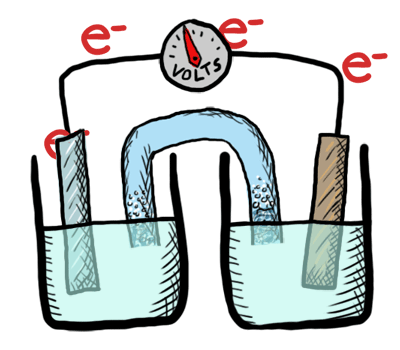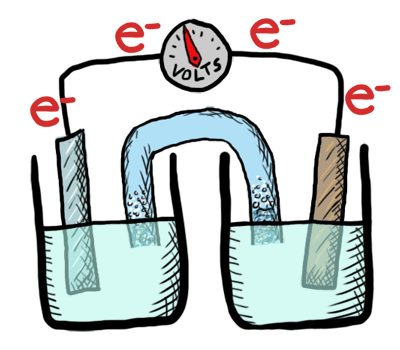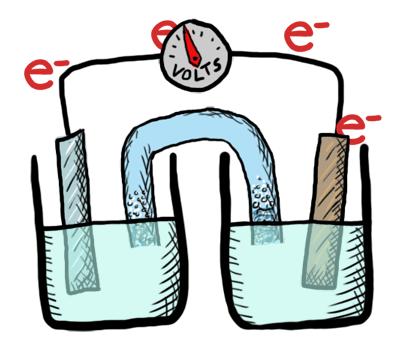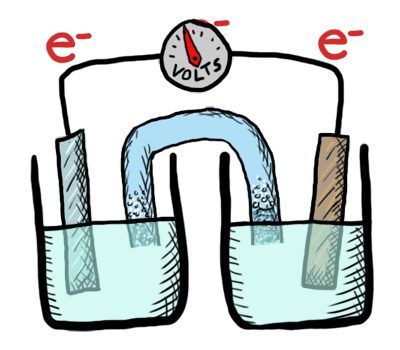
The standard potential for a voltaic cell is positive. The standard free energy for a reaction \(\Delta G^\circ \) is related to the standard potential, \(\mathcal{E}^\circ \), such that negative free energy (spontaneous) corresponds to positive potential. The beauty of electrochemistry and electrochemical cells is that we can now directly measure the free energy difference by measuring electrical potential.
Voltaic cells can be referred to as Galvanic Cells. This is a different word for the identical concept. They are also batteries. Since a battery is an electrochemical cell that produces a voltage (and current) spontaneously, it is a voltaic cell. You will find these three terms used interchangeably.
Below is a picture that summarize the ideas for a voltaic cell.
For a voltaic cell \(\mathcal{E} \gt 0 \), \(\Delta G \lt 0 \). This is the sign convention for spontaneous change.
Electrons always flow from the anode to the cathode in every electrochemical cell (voltaic and electrolytic) by the very definition of what occurs at the electrode surfaces. However, in voltaic cells the process is spontaneous - the electrons are driven through the external circuit by the free energy of the spontaneous redox reactions occurring in each half cell.
The one odd detail is the "sign" of the electrodes. For a voltaic cell the cathode is assigned the "+" sign. Why? Because it is actually drawing in electrons to "feed" the reduction going on. This means the electrode is inherently positive. Also, in this context, cations are migrating towards the cathode in the solution.
In an analogous way, the anode is pushing electrons out and away. Only a negative charge would spontaneously do that, so the anode on a voltaic cell is negative (-).
The "odd" part is that the signs on the cathode and anode in an electrolytic cell are the opposite way, the cathode is negative (-), and the anode is positive (+). This is because electrolytic cells are NOT spontaneous at all, there is often nothing there to push or pull electrons in either direction, like in a salt water solution (say aqueous Na2SO4). Electrons are FORCED upon the electrode that then becomes a cathode. Forcing electrons on an electrode will make it negative (-). Forcefully REMOVING electrons from the other electrode makes it positive and the anode (+). So electrolytic cells have the opposite signs associated with the cathode and anode as they do in voltaic cells.
An electrochemical cell in which the chemistry is non-spontaneous is called a electrolytic cell. This means that the oxidation will not occur spontaneously at the anode and the reduction will not be spontaneous at the cathode. The chemistry we would like to occur is uphill in free energy. It can't happen unless we make it happen. For most chemical reactions, this can only be accomplished by coupling it to another reaction in which the free energy change is sufficiently negative that the two reactions together are spontaneous. In the case of electrochemistry, it is significantly easier as we can simply drive the reaction by attaching an external power supply.
The standard potential for an electrolytic cell is negative. The standard free energy for a reaction \(\Delta G^\circ \) is related to the standard potential, \(\mathcal{E}^\circ \), such that positive free energy (non-spontaneous) corresponds to negative potential. To make the reaction "go," we must apply a voltage that is significant enough to overcome the negative potential of the cell itself (it is spontaneous in the reverse direction).
Electrolytic cells thankfully don't have many names. However processes such as electroplating, or electrodeposition are typically electrolytic cells.
Below is a picture that summarizes the cell nomenclature for a electrolytic cell.
For an electrolytic cell \(\mathcal{E}^\circ \lt 0 \), \(\Delta G^\circ \gt 0\)
Electrons flow from anode to cathode (this is always the case). For an electrolytic cell however, this flow is not spontaneous but must be driven by an external power source.
In an electrolytic cell, the anode has the "+" sign.It is important to realize that an electrolytic cell and a voltaic cell as essentially exactly the same with the exception of what we "want to happen". The chemistry is always spontaneous in one direction. So if we chose a particular direction by calling something a "reactant" or "product" then it may or may not be spontaneous in that direction. Similarly by declaring one electrode the "anode" and one the "cathode" we are choosing where we would like the oxidation and reduction to occur. This may be spontaneous (a voltaic cell) or it may not be spontaneous (an electrolytic cell).
Generally electrolytic cells are used to generate unstable (high free energy) compounds or elements from stable (low free energy) compounds. With electrical work we can force the chemistry from low free energy back up to higher free energy. This can be extremely useful. We can use electricity to generate hydrogen and oxygen (high free energy) from water (low free energy). Or we can use electricity to generate sodium metal and chlorine gas from sodium chloride salt. This can lead to a slightly different physical configuration for an electrolytic cell. In a voltaic cell we need to separate the starting materials (reactants) from one another so that they do not spontaneously react with each other. For many electrolytic cells this is not necessary since the "reactant" is a stable compound like NaCl. However, it is critical for such an electrolytic cell that we separate the products from one another as they will spontaneously recombine. For example, in an electrolytic cell that produces Na(s) and Cl2(g) we start with NaCl(s). To make the cell first the NaCl is melted. Then the anode and cathode are placed into the molten NaCl. At the anode the Cl-1 is oxidized to Cl2 gas and at the cathode the Na+ is reduced to solid Na. These two elements will react with each other spontaneously to reproduce NaCl if they come in physical contact. Therefore, the cell needs to be constructed such that this never happens. Finally, such a cell does not require a salt bridge. This is because the molten NaCl is an ion conductor, and the reaction is removing anions at the anode and cations at the cathode so the charge balance is maintained.
© 2013 mccord/vandenbout/labrake