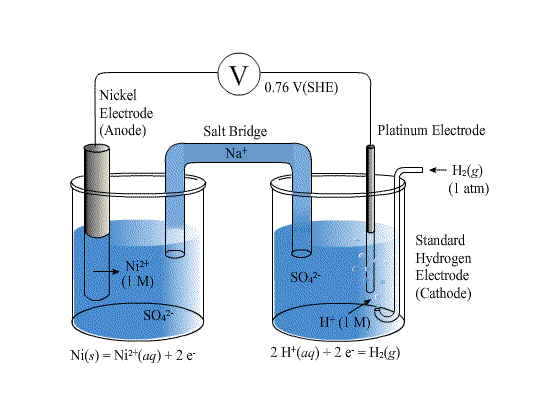
It becomes very cumbersome to draw a picture of an electrochemical cell each time you would like to discuss one.
As such, we have developed a short hand notation for a cell.
For the cell above we have the anode on the left and the cathode on the right. The oxidation is occurring at the anode
\[\rm{Ni(s) \rightarrow Ni^{2+}(aq) + 2e^-}\]
The reduction is occurring at the cathode
\[\rm{2H^+(aq) + 2e^- \rightarrow H_2(g)}\]
The overall reaction is
\[\rm{Ni(s) + 2H^+(aq) \rightarrow Ni^{2+}(aq) + H_2(g)}\]
Rather than having this picture of the cell, we have a shorthand that is the same moving from left to right in our diagram. Starting with the anode on the far left, we write the atomic symbol for the solid material for which the anode is composed. Next we show the aqueous components in the anodic-half of the cell. We separate each component with a "|" symbol (vertical line). All aqueous components of the anodic half-reaction will be listed as part of the aqueous phase. Note that the order within the aqueous phase is not important, we just make sure that all necessary species are listed. Thus for our cell, we only have to list Ni2+ in the aqueous phase because there are no other species in the half-reaction. The anodic half-cell has Ni(s) being oxidized to Ni2+ in solution. Because Ni(s) itself is a conductor (a metal), we can just use it as the anode. In shorthand notation we would write the anode-half of the cell as
\[\rm{Ni\;\bigl|\;Ni^{2+}}\]
Next we would denote the cathode half of the cell. Again we would list all solution species followed by a vertical bar for phase change until we reach the anode material which will be listed on the far right of the notation. Here the reactant is H+, the product is H2, neither of which is a conductor which means an inert electrode must be used which will be Pt (the cathode). Even though our reduction reaction has two moles of H+ for each mole of H2 we do not show any stoichiometry in the shorthand notation - we simply list the reactive species that identifies the right half reaction. So the cathode-half of the cell shown would be\[\rm{ H^+ \;\bigl| \;H_2 \;\bigl| \;Pt}\]
Finally, we put the anode and the cathode sides together separated by the salt bridge. The salt bridge is shown as "||" (double vertical line). Thus the final cell diagram is
\[\rm{Ni\;\bigl|\;Ni^{2+}\;\; \bigl|\bigl|\;\; H^+ \;\bigl| \;H_2 \;\bigl| \;Pt}\]
This is the most generic of notations. To be more specific we might include the concentrations (or pressures) along with the phases.
\[\rm{Ni(s)\;\bigl|\;Ni^{2+} (aq,1M)\;\; \bigl|\bigl|\;\; H^+ (aq,1M) \;\bigl| \;H_2 (g, 1 atm) \;\bigl| \;Pt(s)}\]
Reading this notation we know that "on the left" is the implied anodic reaction which in this case is a nickel metal anode that is oxidizing to Ni2+ in solution. That solution is then connected by a salt bridge to a solution of H+ (acid) that are being reduced to H2 gas at a Pt cathode (the cathodic side, the right side). The left side of the cell shown is always to be written as an oxidation: Ni(s) -> Ni2+(aq) + 2e–. The right side of the cell shown is always to be written as a reduction: 2H+(aq) + 2e– -> H2(g). Also remember that in the shorthand cell notation no stoichiometry is shown at all - just species. You will still have to balance the two half reactions against each other in order to determine the overall cell reaction and how many moles of electrons are transferred per mole of reaction completed.